
Collaborations between Western and Indian companies may provide the best path for offshoring successfully and for developing India's readiness.

Collaborations between Western and Indian companies may provide the best path for offshoring successfully and for developing India's readiness.

As facility closures and layoffs continue, CRO and CMO executives are hoping for a better year, despite few positive signs.

A systematic classification system makes supplier quality management feasible, even if you are dealing with hundreds of suppliers worldwide.

A new analysis highlights growth opportunities and challenges for contract development and manufacturing organizations.

An enterprise-wide quality management initiative is required to maintain supplier quality without sacrificing bottom-line objectives.

Lonza's bid for Patheon makes the contract manufacturing industry re-examine the one-stop manufacturer model.
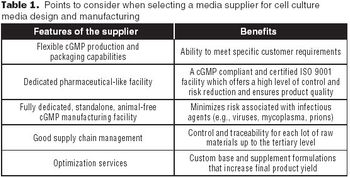
To select the right partner for media design and optimization services, several key factors must be considered.

Contract research, development, and manufacturing organizations betting on a comeback in venture capital financing will have a long wait.

By following key strategies, companies can reduce the risk and increase the benefits of outsourcing analytical development and testing

UK-based CMOs seek opportunities in new markets.

The current competitive environment is forcing service providers to evaluate their business models and focus on value and performance.
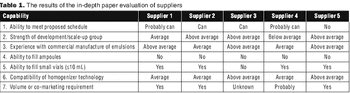
"A systematic approach taken by a company involved making an assessment of internal capabilities, strengths, and needs before the selection process."

What the current lack of venture capital means for the CRO market.

How to ensure smooth technology transfers.
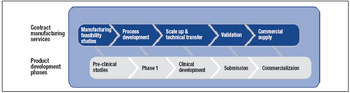
Four reasons why outsourcing may be the best option, and key factors to consider when selecting a provider.

There could be a serious glut of commercial scale mammalian cell culture capacity over the next five years. Then again, there could be a significant shortage. It all depends on how things develop in expression technology, the new product pipeline, and corporate strategies.

Despite the current regulatory uncertainty, pharmaceutical companies should move forward with planning for serialization and pedigree.

Risk mitigation should be a key aspect of any contract manufacturing organization's business strategy.

It is important to understand critical aspects of the CMO's capabilities. Only by auditing certain key areas can the sponsor be assured of the quality of the materials produced.
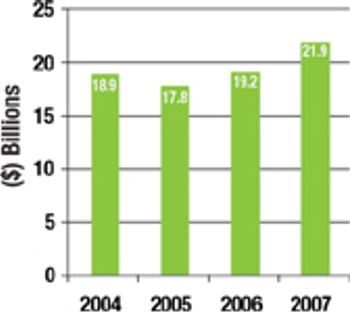
The current overcapacity situation in the bio/pharmaceutical industry is a reminder that CMOs need to come up with business models and value propositions that are based on more than just selling capacity.

There are several steps that you can take to ensure that you get the greatest benefit from consultants.

The supply base for preclinical and clinical development services continues to expand in China.

A CMO faces significant risk of lost revenues and profits if the product fails in clinical trials or doesn't meet sales projections.

China's pharma industry represents 5% of the world total. By 2010, China is expected to become the world's fifth largest pharmaceutical market.
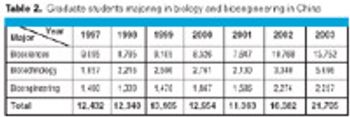
Biotechnology is definitely a hot topic in China-the country's administrators recently identified it as a "cornerstone of China's national economy by 2020." But most realize that getting there will require a better trained, specialized workforce than currently exists. The Chinese government has been pumping money into life sciences education as part of its plan to achieve a global biotechnological presence over the next 15 years.