
The industry is moving beyond cleaning’s “low tech” image to embrace science-based limits and statistical approaches to control.

The industry is moving beyond cleaning’s “low tech” image to embrace science-based limits and statistical approaches to control.

Scaling needs for potential COVID-19 vaccines depend not only on capacity, but also on supply chain challenges and technological hurdles.

Moderna has filed for authorization for its COVID-19 vaccine candidate, which has shown high efficacy and safety in a Phase III trial.

The added space will house a portion of its PVC, polyurethane, silicone, and additional plastic tubing products, as well as its AdvantaPure products.
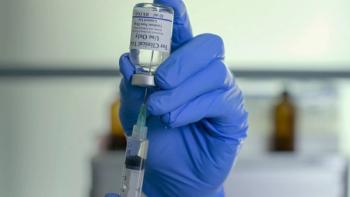
CDMOs strive for flexible, adaptable processes and fast tech transfer for manufacturing investigational drug products, including COVID-19 treatments and vaccines

The expansion is part of a new expansion initiative aimed at increasing flexibility, efficiency, and cost-effective production.

Wacker will support production of CureVac’s COVID-19 mRNA-based vaccine candidate at its biotech site in Amsterdam, with production scheduled to start in the first half of 2021.

The new super plant, being built in Incheon, South Korea, will have a total biopharmaceutical manufacturing capacity of 256,000 L.

The companies have formed a long-term manufacturing agreement to accelerate the global supply for Lilly’s COVID-19 antibody therapeutics.

The agreement follows a recent announcement that Sartorius plans to invest $100 million in Songdo, South Korea’s bio cluster.

The agreement will allow Roche to use CEVEC’s proprietary ELEVECTA technology, which is designed to enable fully scalable, high-performance AAV vector production in suspension bioprocesses.

Apiject was approved for a $590-million loan from the US to build in-country capacity for COVID-19 vaccines and therapeutics.

CordenPharma has announced the expansion of its non-GMP SPPS manufacturing capacity at its Centre of Excellence for Peptide Process Development and non-GMP manufacturing in Frankfurt, Germany.

A second global Phase III clinical trial investigating the safety and efficacy of a two-dose regimen of JNJ-78436735 has been initiated by Johnson & Johnson.

Bone Therapeutics has reported the completion of Catalent’s acquisition of Skeletal Cell Therapy Support SA (SCTS)—Bone Therapeutics’ cell therapy manufacturing subsidiary.

Interim results of AZD1222 trial have demonstrated lower reactogenicity in older adults, and strong immune responses across all adult age groups.

Watson-Marlow Limited Ireland has revealed plans for a new ISO 14644-1 Class 7 cleanroom to be added to the company’s existing site in Cork, Ireland.

Moderna has reported that EMA's CHMP has started a rolling review of mRNA-1273—Moderna’s COVID-19 vaccine candidate.

Pfizer and BioNTech’s BNT162b2 shows efficacy of 95%; the companies plan to submit a request, within days, to FDA for an EUA.

Moderna’s COVID-19 vaccine candidate can be distributed using widely available vaccine delivery and storage infrastructure.

The companies will initially develop and design process technologies, single-use systems, and automation, with plans to eventually focus on an expanding process and digital technologies to optimize a continuous manufacturing process.

FDA issued emergency use authorization for bamlanivimab for patients at risk for severe COVID-19.

Lonza has inaugurated its first ADC payload manufacturing suite at the Visp, Switzerland site.

Sanofi’s Digitally Enabled Integrated Continuous Biomanufacturing Facility in Framingham, MA was named the Overall Award winner at the ISPE virtual annual meeting.

Biopharma can apply new manufacturing practices adopted during the COVID-19 pandemic to enhance bioprocessing.