
Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

Industry experts discuss significant achievements. Plus: What's in store for the future.
The author describes expression technology that produces cell lines with high genetic stability.
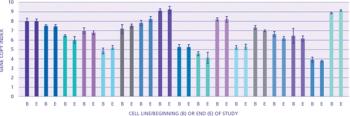
An evaluation of the technologies needed to develop a safe, effective, and economically efficient vaccine. This article is part of a special section on vaccines.
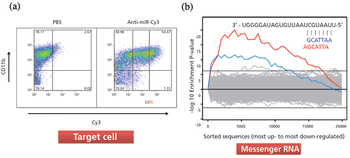
The authors provide insight into microRNA biology, and the simplicity of anti-miR oligonucleotide drug delivery.

Plasmid DNA-encoding proteins offer many advantages, which are now being used in clinical trials.
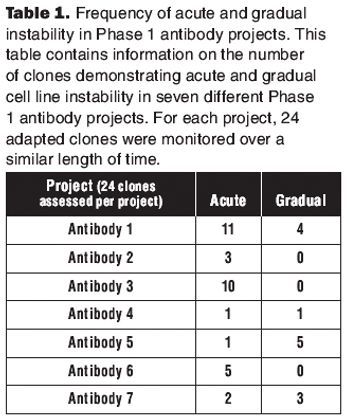
By considering stability as part of the cell line selection and cell banking paradigm, we can ensure that instability problems are not observed during clinical or commercial manufacturing.
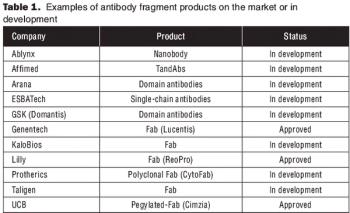
Microbial systems such as E. Coli and yeasts are most effective for producing antibody fragments.
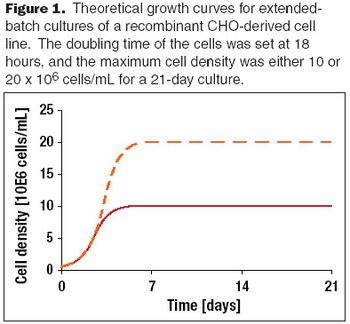
A discussion of past achievements and future expectations of recombinant protein production yields from mammalian cells.