
Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

Microdermics will focus on product development and clinical activities of new drug delivery methods, while Vetter’s primary role will be in the fill and finish aspect.

Operated by BioOutsource, Sartorius�’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.
The unique structures of fusion proteins lead to expression, heterogeneity, and stability issues.

The authors explore the use of precipitation using polyvinyl sulfonic acid and zinc chloride in place of capture chromatography to reduce the cost of goods in the insulin manufacturing process.

Under the terms of the agreement, Sartorius Stedim Biotech will manufacture membrane adsorber technologies for GE, which will be marketed as part of GE’s ReadyToProcess product offerings.

The European Pharmacopoeia Commission re-evaluates its policy on the development of monographs for finished drug products.

Manufacturing standards are considered key to preventing drug recalls and shortages.

A proposed new guideline provides a global policy for limiting metal impurities qualitatively and quantitatively in drug products and ingredients.

The nimbleness of biotechs makes them well suited to implementing QbD. Here's how to get started.
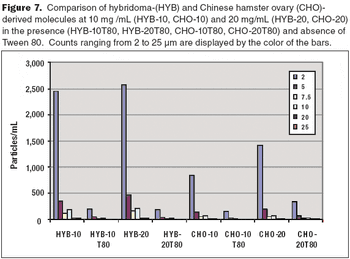
How to maintain product stability and prevent particulates.

The FDA is under attack from all sides. Many influential members of Congress either don't trust the agency to monitor the industry appropriately, or have found it politically expedient to keep sounding alarms about inadequate oversight of food and drug safety and clinical research. The good news is that there seems to be a growing consensus that FDA needs a major infusion of cash to regain its stature as an effective science-based regulatory agency.