
Boehringer Ingelheim joins Oxford BioMedica, UK Cystic Fibrosis Gene Therapy Consortium, and Imperial Innovations to form a partnership for developing a new gene therapy to treat cystic fibrosis.

Boehringer Ingelheim joins Oxford BioMedica, UK Cystic Fibrosis Gene Therapy Consortium, and Imperial Innovations to form a partnership for developing a new gene therapy to treat cystic fibrosis.

The National Science Foundation grant will be used to commercialize a synthetic biology platform for cancer drug development.

Moderna’s new manufacturing plant in Norwood, MA gives the company capacity for preclinical and Phase I and II clinical manufacturing for its mRNA development candidates, including personalized cancer vaccines.

Sanofi will open a R&D operations hub in Chengdu, Sichuan province, China, with a focus on digitalization and big data analysis.

The new company will develop proprietary RNA-based therapeutics and will provide broad lentiviral development and manufacturing expertise and support.

Smaller review divisions will bring experts closer to decision processes and reduce bottlenecks, FDA leaders say.

Scientists at Washington University School of Medicine in St. Louis have developed a new method that could help increase the long-term effectiveness of gene therapy.

Research from Gladstone Institutes suggests that transplanting genetically altered interneurons could improve cognitive function for Alzheimer’s disease.

LNC Therapeutics has appointed a new CEO and is strengthening its R&D for gut microbiome-based drugs.

Roche has acquired a program to develop regenerative therapies for multiple sclerosis.

National Institutes of Health researchers use genomics to show that squamous cell carcinomas differ from other cancers, which could advance treatments for head and neck and other cancers.

Gyros Protein Technologies’ new immunoassay technology includes expanded software to optimize time to results and sample capacity while simplifying workflows.

Biogen will acquire an AMPA receptor potentiator for cognitive impairment associated with schizophrenia in a deal worth approximately $590 million.
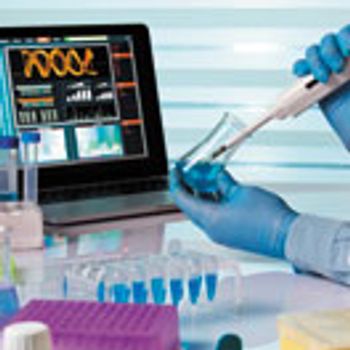
Successful outsourcing relationships for early phase analytics in drug development are driven by partnership.

PharmaMar has released the results of MI130004, a novel antibody drug conjucate created using a molecule of marine origin.

The research partnership will focus on advancing regenerative medicine using porcine bioproducts.

A new report states that more research and clinical development must be done in the treatment of pain and addiction.

The companies will partner to further immuno-oncology research using humanized mice.

The acquisition strengthens Charles River Laboratories’ capabilities in the oncology and immunology therapeutic areas.

The acquisition, valued at EUR 520 million (US$631 million), would expand Takeda’s late-stage pipeline in gastroenterology and would extend an already existing collaboration between the two companies.

A Takeda and Denali collaboration includes three named programs for treating Alzheimer’s disease and other neurodegenerative diseases, using Denali’s antibody transport vehicle (ATV) technology to enhance blood-brain barrier penetration.

The companies aim to discover and develop locked nucleic acid oligonucleotides as orally available therapies for treating inflammatory bowel diseases.

The companies aim to develop a potential zinc finger protein transcription factor-based gene therapy for treating Lou Gehrig’s disease.

Children with a rare brainstem-based cancer might be helped by a new immunotherapy that targets a mutated protein found exclusively in cancer cells.

The acquisition deepens Astellas’ involvement in the development of a pipeline of therapeutics focused on mitochondrial function.