
BioPharm International speaks with a CMO to find out how it is coping with capacity challenges, regulatory authority scrutiny, supply chain reliability, and new requests from clients to incorporate continuous manufacturing into processing lines.


BioPharm International speaks with a CMO to find out how it is coping with capacity challenges, regulatory authority scrutiny, supply chain reliability, and new requests from clients to incorporate continuous manufacturing into processing lines.

The ready-to-fill packaging solutions for vials are based on Ompi EZ-fill packaging design.

Vetter expanded visual inspection facilities and controlled-temperature storage at its Ravensburg Vetter West facility in Germany.

The company expanded its commercial packaging facility in response to a growing demand for pediatric drugs.
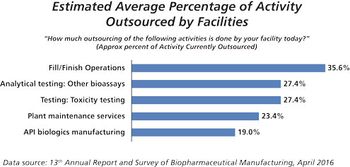
This key bioprocessing segment is expecting continued growth.

GenPak Solutions is cited by FDA for cGMP violations at its Hilliard, Ohio facility.

Real-time GPS technology, better IT connections, and more conservative, controlled shipping temperatures are improving the shipment of sensitive pharmaceuticals.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

The challenge of achieving zero visible defects (i.e., particulates) in parenteral drugs will require a coordinated effort at all stages of the supply chain, particularly in the production and filling of primary containers.

Automation and disposables continue to reduce human error.
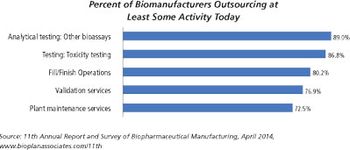
There are significant differences between small molecules and biologics fill/finish capacity.

Aesica Pharmaceuticals S.r.I. collaborates with QAD to meet China's Food and Drug Administration's shortened serialization deadline.

Vetter launches new serialization process to support track-and-trace efforts.

Almac releases an enhanced third-party logistics customer billing application.

BioPharm International speaks with industry experts about challenges faced in managing the cold chain.

A Q&A with SAFC and BioReliance about sourcing and risk mitigation for raw materials.