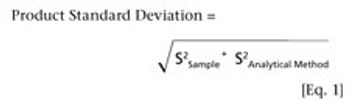
Knowing how method performance impacts out-of-specification rates may improve quality risk management and product knowledge.

Knowing how method performance impacts out-of-specification rates may improve quality risk management and product knowledge.

Experts discuss recent advances in cell viability testing methods in bioreactors.
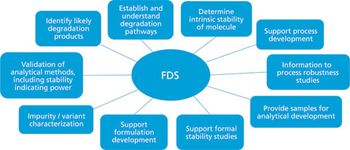
The author addresses critical issues to consider prior to performing forced degradation studies and provides best practice recommendations for these types of studies.
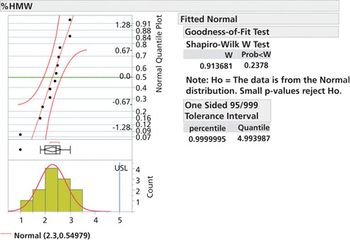
Specification limits should be set early in drug development and refined in later phases as data becomes available.
Data acquired from osmolality, glucose, and folic acid tests provides useful information for the specific identification of cell-culture media.
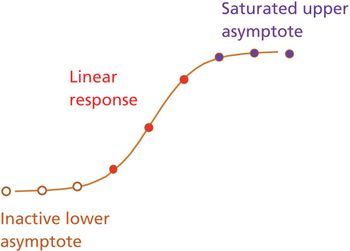
The author describes common components of a relative potency bioassay and provides a framework for assay development, calculation, and control.
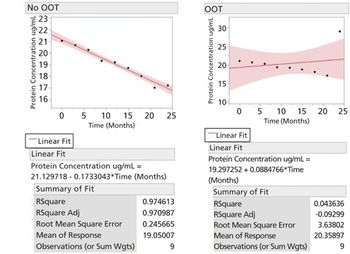
This article defines the concept, justification, and method of removal of out-of-trend points in stability modelling and shelf-life prediction.

Whether taking an upstream, downstream or holistic approach, there are many factors to consider when choosing viral clearance methods.
The authors present a review of the techniques commonly used for glycosylation analysis.
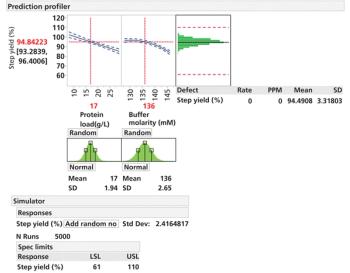
An approach to small-model generation and calibrating small-scale models to reliably predict performance at scale is presented.

imagewerksBiophysical binding studies utilizing surface plasmon resonance, biolayer interferometry, isothermal titration calorimetry, or related techniques are ce
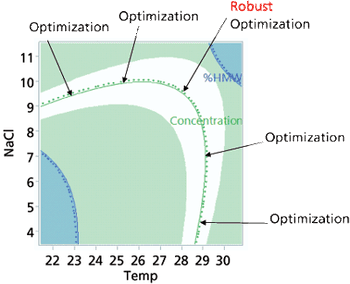
Approaches to the generation of process models, optimization techniques, and application of a design space are explored.

This article reviews factors that affect protein stability at different steps of the product manufacturing process and strategies to minimize their impact on product quality.

The rapid testing of biologic raw materials can lead to greater efficiency.
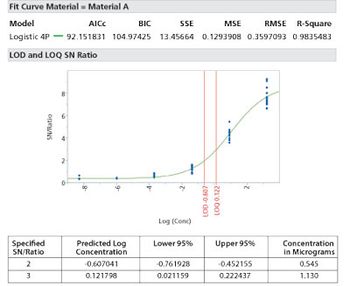
Care needs to be made to match the method of limit determination to the analytical method.
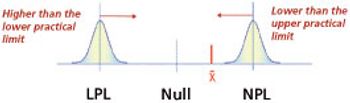
Understanding the influence of change events on product performance is a necessity to routine drug development, transfer, and validation.
The ability to define a scientifically justified and statistically sound sampling procedure is a fundamental skill in modern systematic drug development.
Design space generation is encouraged in new product development.
Characterization of stability performance provides a clear, statistically defendable method for determining accelerated stability.
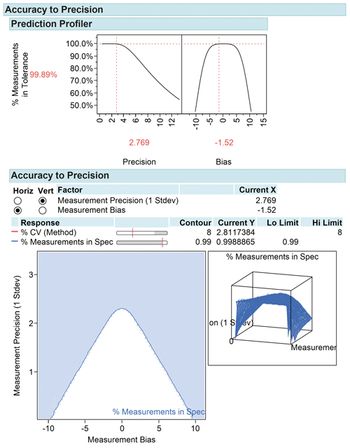
Design of experiment is a powerful development tool for method characterization and method validation.
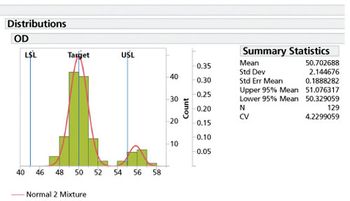
Variation understanding and modeling is a core component of modern drug development.
A well-designed comparability study can demonstrate the performance and advantages that can be gained when adopting a new protocol.

Knowledge of product or process acceptance criterion is crucial in design space.
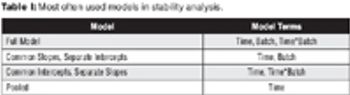
The author discusses the need for stability analysis.