
Express Scripts announced its plan to offer a $1 alternative to Daraprim, a generic drug used for the treatment of toxoplasmosis.

Express Scripts announced its plan to offer a $1 alternative to Daraprim, a generic drug used for the treatment of toxoplasmosis.

FDA should not release naming rules for biosimilars ahead of interchangeability guidelines, says the pharmacy benefit manager.

The agency promotes safer use of drugs and prevention of medication errors through a new webpage and practice guide.

Under the terms of the agreement, Xellia will acquire substantial parts of the Ben Venue site, including four sterile injectable manufacturing plants, which are not currently operational.

FDA seeks industry support for metrics program, emphasizing the surveillance focus.

Chugai Pharmaceuticals, a research-based company headquartered in Tokyo, opens new facility in Berkley Heights, NJ.
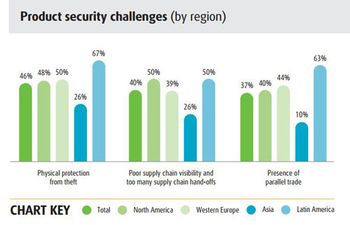
The 2015 UPS supply chain survey suggests that pharma companies need to improve cost control and planning for unexpected events.

Robert Califf addresses questions about drug pricing at the Senate hearing to weigh his appointment to be the next commissioner of FDA.

The new executive director of the European Medicines Agency begins appointment.

A landmark study by the National Institutes of Health determines that Lucentis is highly effective as a treatment for diabetic retinopathy.

An ABPI report found a lack of quality candidates for high-skilled roles in areas such as bioinformatics, translational medicine, clinical pharmacology, and pathology.

FDA seeks feedback on possible analytical standards and approaches to optimize regulation of next-generation sequencing (NGS)-based in vitro diagnostic tests.

Lawsuit alleges birth control packaging error led to 113 unwanted pregnancies.

Even though rising production and use of generic pharmaceuticals is saving billions for the nation’s healthcare system, policy makers continue to slap the industry with policies it claims will limit product development and sales.

The European agency presents guidelines for conducting post-authorization efficacy studies.

According to Diplomat’s CEO Phil Hagerman, true specialty pharmacies are “not in the business of toenail fungus and teenage acne.”

All biosimilars for a specific product will be reimbursed with the same J-code under Medicare Part B regardless of manufacturer, according to a CMS rule that was proposed in July 2015 and finalized on Oct. 30, 2015. The rule was finalized prior to any formal guidance from FDA on interchangeable products. CMS said it did not consider interchangeability into its decision, as there are no currently approved interchangeable biologics on the market.

The “next-generation” design for the pods will build on Pfizer’s existing modular prototype for oral solid-dose manufacturing.

The agency issued a draft guidance document on the requirements for submission of applications for liposome drug products.

USP responds to FDA's draft guidance on the naming of biological products.

Despite considerable investment by biotech manufacturers in developing competitive biologics for the US market, gaining FDA approval of these products has turned out to be a slow and complex process.

According to a third-quarter earnings report, Pfizer’s vaccine business contributed nearly 14% to its total revenue.

Melanoma treatment, Imlygic, received a positive opinion from CHMP, and several other products received extension of indications.

The agency provides terms and recommendations for packaging and labeling of injectable medical products.

The agency gives an update on the regulation of combination medical products.

Celebrating the best of pharma and recognizing companies that turn inspiration into innovation.

Through the Glycoscience Program, the National Institute of Health will contribute $10 million to advance the study of carbohydrates and the compounds that interact with them.

The Cell History File is designed as a “cell passport” for developers and manufacturers of tissue- and cell-based medicinal products.

The agency provides recommendations for submitting proposed labeling with abbreviated new drug applications.

The PDA report discusses qualification and operational handling of passive thermal protection systems.