
ERS Genomics revealed that the European Patent Office (EPO) has rejected arguments filed in opposition to patent EP2800811, which is directed to the single-guide CRISPR/Cas9 gene editing system and covers uses in cellular and non-cellular settings.

ERS Genomics revealed that the European Patent Office (EPO) has rejected arguments filed in opposition to patent EP2800811, which is directed to the single-guide CRISPR/Cas9 gene editing system and covers uses in cellular and non-cellular settings.

The Native Antigen Company has commercially introduced antigens that have been specifically derived from the Wuhan strain of novel coronavirus, now named Covid-19.

The report details OPQ’s accomplishments over the past five years.
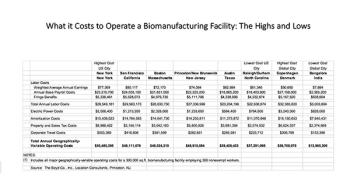
As politicians focus on drug cost reduction, biopharmaceutical companies in the US are moving to states with lower taxes, and relocating some facilities that had been offshore.

The research community is moving quickly to launch clinical trials of potential countermeasures, while regulatory authorities aim to support product development through regulatory flexibility.

FDA published draft guidance for applicants seeking licensure of a proposed biosimilar or proposed interchangeable biosimilar.

The two agencies are collaborating to support a robust biologics marketplace by taking steps to deter anti-competitive business practices.

The FDA Commissioner plans to address drug prices, the drug approval process, and supply chain issues during his time as commissioner of FDA.

The vaccine is designed to provide active immunity against the influenza A (H5N1) strain and can be easily deployed in a pandemic event.

The UK BioIndustry Association (BIA) has issued a statement welcoming the publication of the Life Sciences 2030 Skills Strategy, a report setting out how the life sciences sector in the United Kingdom will develop future talent.

WuXi’s R&D team will be developing and manufacturing 2019-nCoV-neutralizing antibodies at the company's four GMP facilities.

The agency has published seven guidance documents directed at the development and manufacture of gene therapies.

The agency celebrates the efforts it has made in creating a system for the evaluation and supervision of medicines throughout the European Union.

The FDA guidance provides an explanation of changes to user fees under the Biosimilar User Fee Amendments of 2017 under Title IV of the FDA Reauthorization Act of 2017.

As the facility becomes fully operational, the company believes the potential risk of a shortage of the product due to increasing demand will be significantly reduced.

The new course is directed at analyzing the skills gap in the manufacture of cell and gene therapies as they progress toward manufacturing at scale.

The drug treats adult patients with secondary progressive multiple sclerosis with active disease evidenced by relapses or imaging features of inflammatory activity.

The agency’s joint Big Data Task Force and the Heads of Medicines Agencies proposed actions for the use of big data to support innovation and public health.

FDA sent a warning letter to Health Pharma USA after an inspection found the company’s quality unit was not properly overseeing its drug manufacturing operations.

The formation of the new gene therapy company stems from the progress and success of Nationwide Children’s Hospital’s clinical manufacturing and gene therapy work.

FDA has granted fast track designation for Novavax’s NanoFlu, a recombinant quadrivalent flu vaccine, for use by adults age 65 years and older.

Horizon Discovery Group and Mammoth Biosciences have signed a collaboration and license agreement aimed at the development of the next generation of engineered Chinese hamster ovary (CHO) cell lines to improve biotherapeutics production.

The Cell and Gene Therapy Catapult (CGT Catapult) has revealed that the United Kingdom is a favorable environment for cell and gene therapy clinical trials.

ProBioGen and Lava Therapeutics have closed the cell line development and manufacturing agreement for Lava’s novel bispecific antibody lead candidate.

Top of CDER’s to-do list for 2020 is tracking adverse events more effectively and combating the opioid crisis.

Can a commitment to limit prices to ensure patient access to important new medicines regain public trust and confidence in the biopharmaceutical industry?

The trial will test experimental stem-cell treatments against biologic therapies for severe forms of relapsing multiple sclerosis.

The company has various projects and launches scheduled for 2020 as part of its anniversary celebration.

Tim Howard will assume the role of acting ISPE CEO and president after John Bournas steps down from the positions.

The numbers of new molecular entities approved in 2019 are close to or exceed FDA’s performance in most previous years.