
Infrastructure and payer decisions will determine drug choices in emerging and developed regions.

Infrastructure and payer decisions will determine drug choices in emerging and developed regions.

GlaxoSmithKline acquires Bristol-Meyers Squibb’s late-stage HIV R&D assets.

This month marks the death of a pioneering, yet pragmatic, thinker whose work brought PAT and QbD to more pharmaceutical and biopharmaceutical companies around the world.

Synpromics announced collaborations with Avalanche Biotechnologies and Applied Genetic Technologies Corporation to use synthetic promoters to develop gene therapies, including adeno-associated virus technology for treating eye diseases.

The aim of the collaboration is to advance the use of Cellectar’s phospholipid drug conjugate platform for targeted delivery of a selection of Pierre Fabre’s cytotoxics.

AstraZeneca announced the completion of a tender offer for all of the outstanding ZS Pharma shares.

Through a strategic alliance with WuXi AppTec and a $50 million facility investment, AstraZeneca announces biologics and small-molecule expansion in China.

AstraZeneca partners with the Wallenberg Center for Protein Research to conduct studies on the Secretome.

Sanofi Pasteur was granted marketing authorization for Dengvaxia, the first vaccine for dengue fever.

On Dec. 8, 2015, Sandoz, the generic pharmaceutical’s division of Novartis, announced that the European Medicines Agency (EMA) has accepted their marketing authorization application (MAA) for a biosimilar to Enbrel (etanercept), a tumor necrosis factor alpha (TNF-alpha) inhibitor.

Amgen submitted a market authorization application to EMA for ABP 501, a biosimilar candidate to Humira.

TxCell signs strategic agreement with MaSTherCell for European manufacturing of its cell therapy products.

Adaptimmune Therapeutics and Universal Cells enter into collaboration and license agreement to develop universal allogeneic T-cell therapies.

Seqirus, CSL Limited’s influenza vaccine business, announced the opening of their corporate headquarters in the United Kingdom.

Express Scripts announced its plan to offer a $1 alternative to Daraprim, a generic drug used for the treatment of toxoplasmosis.

Amgen announced the submission of a BLA with the FDA for ABP 501.

The Committee on Energy and Commerce met to assess influenza vaccine effectiveness, and changes for the 2015-2016 season.

Paras Biopharmaceuticals and Novozymes will collaborate on the creation of an improved osteoporosis treatment.

The portal is an interactive tool that pulls together the innovation landscape, company base, and manufacturing sites for the entire medicines sector in the UK.

Under the terms of the agreement, Xellia will acquire substantial parts of the Ben Venue site, including four sterile injectable manufacturing plants, which are not currently operational.

Chugai Pharmaceuticals, a research-based company headquartered in Tokyo, opens new facility in Berkley Heights, NJ.
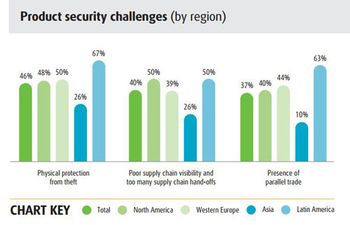
The 2015 UPS supply chain survey suggests that pharma companies need to improve cost control and planning for unexpected events.

BioOutsource releases informational video detailing issues associated with ADCC assays and how to effectively analyze them.

A landmark study by the National Institutes of Health determines that Lucentis is highly effective as a treatment for diabetic retinopathy.

FDA grants accelerated approval for Darzalex (daratumumab) for the treatment of multiple myeloma.

A new consortium involving Arecor, FUJIFILM Diosynth Biotechnologies and the Center for Process Innovation will focus on formulation innovation as a way to improve downstream processing and reduce biopharmaceutical cost.

High-dose axalimogene filolisbac immunotherapy will advance to expansion phase.

The University of Pittsburgh partners with biopharmaceutical company, Shire plc, to research rare diseases.

As a result of the acquisition, Merck will have access to Harrisvaccine’s proprietary RNA Particle technology production platform.

A study and new index shows that only 65% of clinical trials are being registered and reported on. Nearly half of the new drugs approved by FDA in 2012 had one Phase II or III trial that was not disclosed. Regulatory ambiguities, mergers, and lack of enforcement action may be to blame