
The companies are entering into a development and manufacturing collaboration for the advancement and production of human monoclonal antibodies for the potential treatment of novel coronavirus.

The companies are entering into a development and manufacturing collaboration for the advancement and production of human monoclonal antibodies for the potential treatment of novel coronavirus.

Takeda announced the acquisition after the conclusion of a Phase 1 study of the investigational medicine TAK-062 (Kuma062) for the treatment of uncontrolled celiac disease.

If approved, the therapy may become the first-choice treatment for relapsing multiple sclerosis patients and will be the first B-cell therapy that can be self-administered using an autoinjector pen.

The partnership will begin in the second half of 2020.

FDA has granted breakthrough therapy designation to padcev (enfortumab vedotin-ejfv) in combination with Merck’s anti-PD-1 therapy keytruda (pembrolizumab).

The companies aim to develop novel cell therapies for treating multiple cancers.

Sanofi will use a recombinant DNA platform that produces a genetic match to proteins found on the surface of COVID-19 to formulate the vaccine.

If approved, this treatment will be the first therapy targeted for METex14-mutated advanced lung cancer.

ERS Genomics revealed that the European Patent Office (EPO) has rejected arguments filed in opposition to patent EP2800811, which is directed to the single-guide CRISPR/Cas9 gene editing system and covers uses in cellular and non-cellular settings.

The Native Antigen Company has commercially introduced antigens that have been specifically derived from the Wuhan strain of novel coronavirus, now named Covid-19.

Through the agreement, Catalent will offer process optimization and drug substance manufacturing services for the drug candidate at its Madison, WI site.
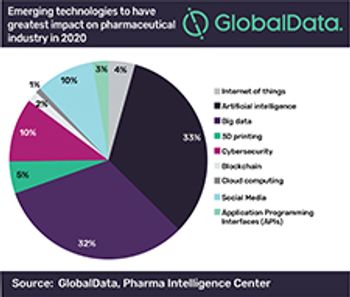
A growing number of pharma executives see investment in machine learning and big data as a top priority, according to a 2020 GlobalData survey.

Aimmune plans to introduce the antibody as an adjunctive treatment with its Characterized Oral Desensitized ImmunoTherapy programs to research treatment outcomes in patients with food allergies.

The vaccine is designed to provide active immunity against the influenza A (H5N1) strain and can be easily deployed in a pandemic event.

Abcam has purchased Applied StemCell’s (ASC’s) gene editing platform and oncology product portfolio, adding comprehensive cell editing capabilities and engine to support expansion of existing “off-the-shelf” cell lines.

Otsuka will use PhoreMost’s phenotypic screening platform to identify new targets for drug development, with a focus on gene therapy.

The license gives Daiichi Sankyo access to ERS Genomics’ genome-editing technology for internal R&D initiatives.

J&J’s Janssen Pharmaceutical Companies seeks to develop a vaccine candidate against the novel coronavirus.

Clinical manufacturing of the therapy will take place at Lonza's Houston, TX, and Netherlands cGMP manufacturing sites.

As the facility becomes fully operational, the company believes the potential risk of a shortage of the product due to increasing demand will be significantly reduced.

The drug treats adult patients with secondary progressive multiple sclerosis with active disease evidenced by relapses or imaging features of inflammatory activity.

FDA has granted fast track designation for Novavax’s NanoFlu, a recombinant quadrivalent flu vaccine, for use by adults age 65 years and older.

The drug is formulated to improve cardiac contractility with a reduced effect on heart rate, blood pressure, and myocardial oxygen consumption while potentially avoiding adverse events associated with current inotrope therapies.

Horizon Discovery Group and Mammoth Biosciences have signed a collaboration and license agreement aimed at the development of the next generation of engineered Chinese hamster ovary (CHO) cell lines to improve biotherapeutics production.

The Cell and Gene Therapy Catapult (CGT Catapult) has revealed that the United Kingdom is a favorable environment for cell and gene therapy clinical trials.

ProBioGen and Lava Therapeutics have closed the cell line development and manufacturing agreement for Lava’s novel bispecific antibody lead candidate.

The approval comes after the results of a clinical trial that showed 61% of patients had a response lasting six months or longer.

The therapy is currently approved in the EU as a gene therapy for the treatment of patients 12 years and older with transfusion-dependent β-thalassemia.

The approval was based on successful data from a years-long trial that assessed patient’s tumor status every 12 or 24 weeks for up to 24 months.

Under the agreement, Almirall will leverage WuXi Biologics’ proprietary WuXiBody platform to develop bispecific antibodies for dermatological diseases.