
Higher wages and employment rates give biopharm professionals an edge over counterparts in other industries.

Higher wages and employment rates give biopharm professionals an edge over counterparts in other industries.

Ongoing changes create new opportunities for CROs and CMOs.

A prototype Protein A resin is evaluated for purification performance, reusability, and cost performance.

The European Union is strengthening its pioneering role in the regulation of biosimilars by further developing the basic rules for determining the levels of compatibility for this group of drugs. There are, however, some key issues that are not easy to resolve, as evident in a recent workshop on biosimilars organized by the European Medicines Agency (EMA).
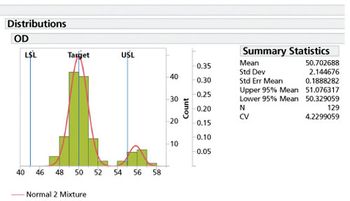
Variation understanding and modeling is a core component of modern drug development.

New policies and products seek to maintain access to pain medicines while curbing rampant abuse.

Wolfgang Weikmann of Vetter Pharma discusses the implementation of quality by design in sterile manufacturing.

Israel's diverse population, high-quality healthcare system, and resilience to global financial stress make it a strong partner for R'D, clinical research, and market growth.
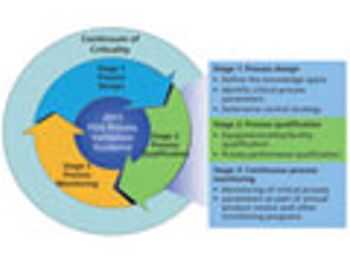
Criticality is used as a risk-based tool to drive control strategies.

The employment picture brightened a bit, but biopharma employees still seek better compensation.

The rising cost of drug development and the decreasing proportion of drug-naive population in the US and European markets are driving international pharmaceutical companies to consider emerging markets as a location to conduct their clinical trials. Asia stands out among the emerging markets given its double-digit growth rates.

Click the title above to open the BioPharm International December 2013 issue in an interactive PDF format.