Connectors are a critical element in the process optimization of single-use bioprocessing systems.

Connectors are a critical element in the process optimization of single-use bioprocessing systems.

FDA seeks to focus on problematic facilities and inform firms quickly about site problems.
Despite the disappointing therapeutic performance of ADCs thus far, the pipeline still boasts promising prospects.

The level of formality in change control may be holding back your SOP progress, according to Siegfried Schmitt, principal consultant at PAREXEL.
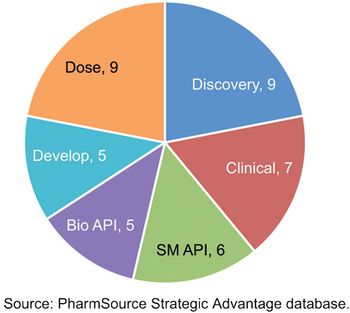
Recent acquisitions are creating CDMOs with scale that rivals global bio/pharma.

Cole Parmer's Masterflex L/S Cytoflow pump head is suited for use in biopharma and microbiology applications.
Qosina's Y connectors are manufactured from plastisol and produced through a dip-molding process.
An updated version of Shimadzu’s LabSolutions analytical data system features an operating environment for complete data management and security in networked laboratories.

The Almac Pod, a temperature-controlled shipping solution service for biologics and other temperature-sensitive products, is compliant with good distribution practices and comes in three models.

Rinse sample analysis or visual inspection are risk-based approaches that can be correlated to surface cleanliness to replace surface sampling in a biopharmaceutical equipment cleaning process.

CPhI Pharma Awards honor companies and individuals driving the pharma industry forward.
Anna Kireieva/Shutterstock.comSpeed to market is essential in the biopharmaceutical industry today.

Despite GxP and data-management challenges, pharma is moving toward new models for clinical trial logistics.
Siliconization is a key process step in the manufacturing of prefilled syringe systems.

Click the title above to open the BioPharm International November 2017 issue in an interactive PDF format.

The use of external retainers to enhance the seal between connectors and tubing is an essential component in single-use manufacturing systems.