
Let's be frank. Love doesn't make the world go round —money does, especially if you're developing a promising technology but you're facing ten or fifteen more years of bench time and clinical trials before you can unveil a marketable product.

Let's be frank. Love doesn't make the world go round —money does, especially if you're developing a promising technology but you're facing ten or fifteen more years of bench time and clinical trials before you can unveil a marketable product.

We've gone beyond the days when corporate America's idea of a healthy outing was a company softball game complete with beer and hot dogs.

Establishing bioequivalence is difficult for drugs with high inter-subject variability or strong dependence on the physiological state of the gut.

Even if FDA does not require pharma companies to co-market a diagnostic test, insurers and health plans may pressure industry to do so, according to Robert Temple of CDER.

Though still relatively modest by international standards, the Netherlands' biotechnology industry has made impressive gains in recent years and now provides investors with a compelling alternative to more established European "heavyweights" such as Scandinavia, Switzerland, and the United Kingdom.

The relationship between "valid" or "suitable and validated" is often overlooked, but there is a high price when "validated" test systems are simply inappropriate.

Wyeth BioPharma has identified cycle-time reduction as critical to customer responsiveness and the success of commercial and pipeline products. In formulating a plan of attack, the company focused on two aspects of cycle time: the global planning process and disposition cycle times.
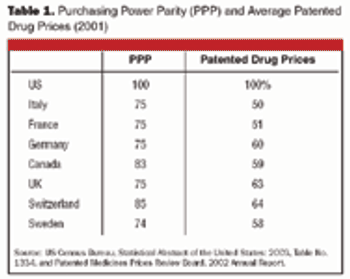
In general, people in other countries pay less for brand-name prescription drugs than people in the US. As a result, some Americans travel to Canada or Mexico to get their prescriptions, and an increasing number are using the Internet to buy drugs from other countries.

Identifying issues in the factory that traditionally arise in the field minimized onsite equipment rework and subsequent qualification work.
A handful of facilities making 200 million single-dose units per year could fast-track the immunization of the developing world.