
FDA works with industry on strategies for assuring high-quality regenerative medicines.

FDA works with industry on strategies for assuring high-quality regenerative medicines.

The differences between the quality control and quality assurance units can be found in their names, according to Siegfried Schmitt, principal consultant at PAREXEL.

Modeling tools help process engineers optimize a biopharmaceutical facility’s capacity.

Faced with divisive political and social issues, Congress must find a way to reach consensus.

Single-use and single-pass TFF devices are facilitating advances in biopharma manufacturing.

Choosing a suitable material for fill/finish containers begins during the product development stage.

Internet of Things, advanced analytics, and blockchain solutions such as smart contracts promise to give manufacturers more control over products and supply chains.
The control of biologics microbiological impurities, contaminants, and mimetics is evolving.

The authors review how media components modulate the quality of monoclonal antibody products
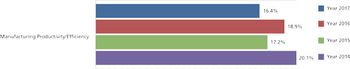
Innovation speeds discovery, drives down costs, and improves productivity.
Protecting against microbiological contaminationover the whole manufacturing process grows increasingly important.

Click the title above to open the BioPharm International September 2017 issue in an interactive PDF format.