This technology includes an efficient and scalable in vitro enzymatic amidation step for peptide hormones.

This technology includes an efficient and scalable in vitro enzymatic amidation step for peptide hormones.

The debate over drug importation is a prime example of politics trumping science as members of Congress reject the consensus of scientists and policy experts that opening US borders to therapies from abroad raises serious public health concerns.

Although there are many differences between the industries, especially related to regulatory requirements, there are enough similarities that the future of biopharmaceuticals with respect to contract manufacturing might look much like the semiconductor industry.
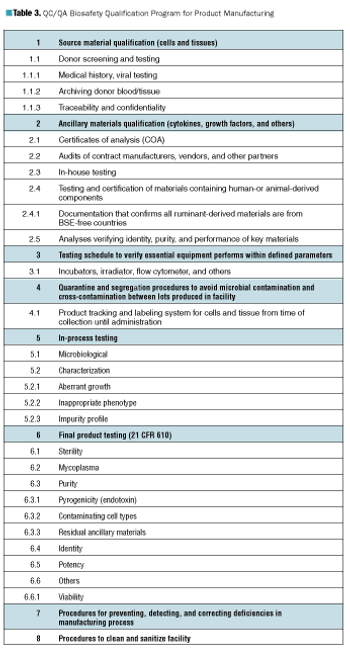
Is it safe? Answering that question for therapies based on living cells is not simple.

The employer's first step in considering whether to use non-competition and non-disclosure agreements is to assess, analyze, and understand the benefits of such agreements.

According to a 2002 report by the consulting firm BioPerspectives, the protein biochip market will reach $700 million in sales by 2006.
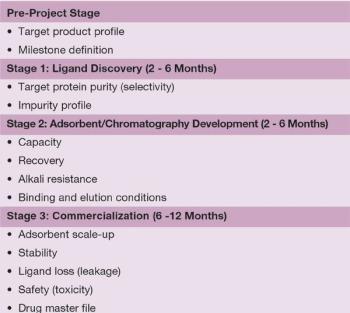
Very significant progress has been made since the mid-1970s when dye ligands were first introduced.

The approaches taken to containing the risk of BSE reveal patterns in the difficulties of performing and reacting appropriately to risk analysis.

Commercial embodiments of genetically modified inventions are protected in Canada.