
Better understanding and control of cell behavior is yielding benefits, upstream and beyond.

Better understanding and control of cell behavior is yielding benefits, upstream and beyond.

The future of raw material sourcing for mAb production may lay in the sustainability of the source and the added benefits of newer technologies.

Ensuring the quality of data in process monitoring and control systems starts in process development phases.

Single-use and modular systems will meet demand for rapid implementation at different scales.

As compounds become more complex in nature and biological ingredients are more widely used, stability testing approaches must follow suit and provide flexibility for developers.

While new industry guidance documents issued by FDA speak to the agency’s efforts to promote the development of new gene therapies, certain hurdles remain to challenge stakeholders.
As patent disputes within the scientific community continue, drug developers consider the intellectual property unknowns associated with this emerging technology.

Contract service organizations can offer biopharma companies early insight into dangers that may hinder a drug’s later development.

States, hospitals, and insurers support manufacturing arrangements to ensure access to affordable medicines.

Emergency actions to protect patients and the drug supply may have long-term implications.
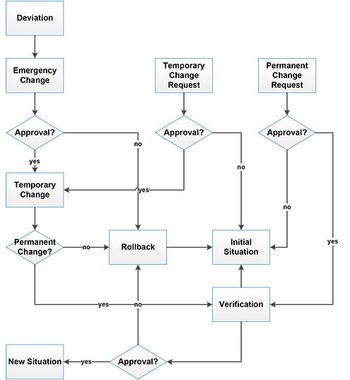
No matter why change may be needed, it is important to comply with all the relevant regulatory requirements, says Siegfried Schmitt, PhD, vice-president, technical, Parexel Consulting.

Click the title above to open the BioPharm International March 2020 issue in an interactive PDF format.