
Changes in the country’s political landscape may affect the pharmaceutical industry market in the future.

Changes in the country’s political landscape may affect the pharmaceutical industry market in the future.

They may not be glamorous, but buffers play an important role in biopharma manufacturing.

Protos 3 is Synbiosis’ automated colony counter and chromogenic identification system.

Watson-Marlow’s 120 cased peristaltic pumps are designed for single-use systems that require no contamination.
As ADCs move through the drug-development process, different analytical methods are often required.

Initiatives to speed drug development must pass Congress and special interest groups.

Evaluating the assembly design process, manufacture, and use helps mitigate risk.

Manufacturers face new rules for tracing drugs through the supply chain and compounders face stricter standards.

Market forces may limit the success of CMOs.
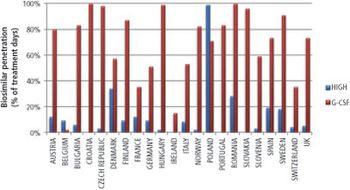
Investors are lining up for the biosimilars market as patents reach expiration and regulatory pathways are defined.
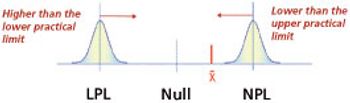
Understanding the influence of change events on product performance is a necessity to routine drug development, transfer, and validation.

EMA is under pressure to exert even tighter standards on biosimilars being marketed in Europe.
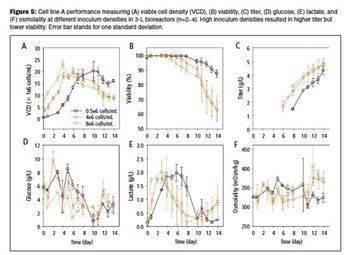
The use of commercially available media to achieve high titer in early process development is discussed.

The author discusses the various ways in which a quality-by-design program can enhance the extractable and leachable assessment of a drug product.

Click the title above to open the BioPharm International February 2015 issue in an interactive PDF format.