
Industry experts discuss the standout packaging trends from 2025 that will influence the industry’s future.
Susan Haigney is lead editor of BioPharm International®.

Industry experts discuss the standout packaging trends from 2025 that will influence the industry’s future.

The company hopes the rebranding effort will better align with its work in cell culture media manufacturing.

EMA Director sees new legislation as an opportunity to revamp policies.

In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates (RCA), and Siegfried Schmitt, Parexel, tackle the ever-growing problem of having a properly trained workforce with a variety of needed skill sets.

Manufacturing biologics is a complex task. Mitigating risk early in the design of manufacturing facilities and the qualification of equipment minimizes future complications.

In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates (RCA), Siegfried Schmitt, Parexel, and Anita Michaels, RCA, explain how CDMOs can best handle regulatory inspections and client expectations.

Eric Hill, Chief Scientific Officer, BA Sciences, discusses the how E&L testing principles can be adapted to biologic products and the unique challenges biologic drugs face regarding E&Ls.

Eric Hill, Chief Scientific Officer, BA Sciences, discusses the analytical challenges involved in conducting E&L testing for biologic drug products.

Vishal Mukund Sonje, Vaccine Manufacturing Lead, CEPI, talks about the challenges that arise in the manufacturing of vaccines in various global regions and gives a preview of his presentation at CPHI Frankfurt 2025.

Pharmaceutical Technology® spoke with Tara Dougal, Event Director for Pharma at Informa Markets about what attendees at CPHI Frankfurt should expect this year.

In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates, and Siegfried Schmitt, Parexel, give their opinions on why those working in the pharmaceutical industry should lend their voices to draft regulations.
Michael Ritchie, chief commercial officer at Champions Oncology, explains what makes radiopharmaceuticals unique in the treatment of cancer.

In this episode of the Ask the Expert video series, Susan J. Schniepp, Regulatory Compliance Associates, and Siegfried Schmitt, PhD, Parexel, answer questions on the use of real-world evidence for both small-molecule and large-molecule drug development. In addition, they tackle a question on supply chain security problems that arise during transportation of pharmaceutical goods.
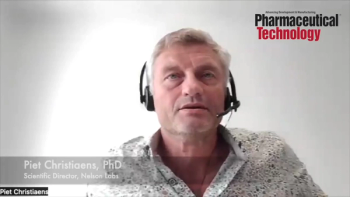
In this episode of the Ask the Expert video series, Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates, talks to Piet Christiaens, PhD, scientific director, E&L expert at Nelson Labs, and Dennis Jenke, PhD, principal consultant for Nelson Labs and chief executive scientist, Triad Scientific Solutions, about extractables and leachables and the testing required for each.

The biological reagents provider is moving to a 5000-square-foot site in Cambridge Technopark to accommodate the company’s growth.

The licensing agreement between the two companies gives Pfizer the rights to develop, manufacture, and commercialize 3SBio’s bispecific antibody, SSGJ-707, which is in clinical trials for the treatment of a variety of cancers.

In this episode of the Ask the Expert video series, Susan J. Schniepp, distinguished fellow at Regulatory Compliance Associates, and Siegfried Schmitt, PhD, vice president, Technical at Parexel, discuss if and when identity testing is necessary to be performed on a final product when the API is sourced from a third party.

The agency has extended the review period for GSK’s biologics license application for belantamab mafodotin-blmf for the treatment of relapsed/refractory multiple myeloma.

The investment is part of CGT Catapult’s Cross-Catapult Investment Pilot and will accelerate the pre-clinical development of Spliceor’s trans-splicing gene therapy platform.

Genascence’s first-in-class gene therapy blocking interleukin 1, GNSC-001, will enter a Phase IIb/III study in 2026.

The company’s gene therapy, AAVB-039, for the treatment of Stargardt disease progresses to a Phase I/II clinical trial.

Northway Biotech will develop and scale production of AATec’s ATL-105 for the treatment of non-CF bronchiectasis.

Scientists at MIT and other institutions have discovered compounds that activate a defense pathway inside host cells that could be used as antiviral drugs.

FDA will review GSK’s application to expand the use of its respiratory syncytial virus vaccine, Arexvy, to adults aged 18–49 who are at increased risk.

The consortium will focus on the delivery of a fully automated robotics cell and gene therapy manufacturing platform.

The next-generation monoclonal antibody could potentially be used to prevent and treat active COVID-19 infections, according to its developer, providing a non-vaccine option.

In this episode of the Ask the Expert video series, Simona Guidi, Associate Director, Cell and Gene Therapies at ProPharma, discusses the options available for controlling adventitious contamination in cell processing and the use of closed processing systems for cell therapy production.

EDQM is providing the 12th edition of the European Pharmacopoeia as an all-digital, redesigned, user-friendly issue.

The agency has recommended conditional marketing authorization for Zemcelpro (dorocubicel/unexpanded umbilical cord cells) to treat adults with hematological malignancies (blood cell cancers).

The Purolite AP+50 affinity chromatography resin has a 50-micron bead size that offers dynamic binding capacity of the AP resin platform while providing durability for monoclonal antibody capture.
Published: October 21st 2025 | Updated: October 22nd 2025

Published: February 1st 2024 | Updated:

Published: October 4th 2024 | Updated:

Published: March 15th 2022 | Updated:

Published: May 15th 2022 | Updated:

Published: March 1st 2023 | Updated: