
Shire's Human Genetic Therapies team, based in Cambridge, MA, specializes in discovering, developing, and commercializing protein therapeutics primarily for the treatment of genetic diseases.

Shire's Human Genetic Therapies team, based in Cambridge, MA, specializes in discovering, developing, and commercializing protein therapeutics primarily for the treatment of genetic diseases.

Acambis plc (Cambridge, UK, www.acambis.com) has announced that the Vaccines and Related Biological Products Advisory Committee of the US Food and Drug Administration has voted unanimously that Acambis' ACAM2000 is both safe and efficacious.

The Bio Affinity Company BAC BV (Naarden, the Netherlands, www.bac.nl) has entered into an agreement with Sanofi Pasteur (Lyon, France, www.sanofipasteur.com), the vaccines arm of the Sanofi-Aventis Group, for the use of BAC's CaptureSelect customized affinity ligands for process-scale purification of some of its vaccine products.

Eden Biodesign (Liverpool, UK, www.edenbiodesign.com), SAFC (St. Louis, MO, www.sigmaaldrich.com/SAFC/Pharma), Midatech Group (Oxfordshire, OX, www.midatechgroup.com), Cellexus Systems (Cambridgeshire, UK, www.cellexusbiosystems.com), and BioConvergence LLC (Bloomington, IN, www.bioc.us) are sprucing up their product development and services with the construction of new manufacturing facilities.

Viragen, Inc. (Plantation, FL, www.viragen.com), and its collaborative partners in the field of avian transgenics-Roslin Institute (Scotland, UK, www.roslin.ac.uk) and (Oxford, UK, www.oxfordbiomedica.co.uk)-have announced a significant breakthrough in the development of the OVA system, an avian transgenic protein expression technology.

Kenneth J. Martin, Wyeth's (Madison, NJ, www.wyeth.com) chief financial officer and vice chairman, has announced plans to leave the company at the end of June.

Millipore Corporation's disposable mixer is designed to help companies mix pharmaceutical ingredients and prepare cell culture media.

Governor Deval Patrick of Massachusetts has proposed a plan for committing $1-billion over the next decade to life-sciences research, including the creation of a stem-cell bank, in an effort to make the state a global leader in biotechnology.

Focetria, a new human vaccine from Novartis (Basel, Switzerland, www.novartis.com) designed for use following the declaration of an influenza pandemic, has received European Union (EU) approval in all 27 member states as well as Iceland and Norway.

James M. Cornelius, who has served as interim chief executive for the past eight months, has been elected chief executive officer of Bristol-Myers Squibb Company (New York, NY, www.bms.com) with a term through the date of the company's annual meeting of stockholders in 2009.

AstraZeneca (London, UK, www.astrazeneca.com) has entered into a definitive agreement to acquire MedImmune, Inc. (Gaithersburg, MD, www.medimmune.com), in an all-cash transaction.

Major pharmaceutical and biotechnology companies have joined forces under the auspices of the Society for Biological Engineering (SBE, New York, NY, www.aiche.org/SBE) to make use of cell line research that is expected to increase the efficiencies of drug development.

Roche (Basel, Switzerland, www.roche.com) has acquired Therapeutic Human Polyclonals, Inc. (THP, Mountain View, CA, www.polyclonals.com), a privately-owned biotechnology company based in California and Germany.

In March, the US Food and Drug Administration released a new draft guidance, along with its guidance agenda for the year.

DelSite Biotechnologies, Inc. (Irving, TX, www.delsite.com), a wholly-owned subsidiary of Carrington Laboratories, Inc. (Irving, TX, www.carringtonlabs.com), has entered into a non-exclusive technology evaluation agreement with Nastech Pharmaceutical Company, Inc. (Bothell, DC, www.nastech.com), for the purpose of evaluating DelSite's GelSite polymer for enhancing the intranasal delivery of peptide and protein therapeutics.

The FDA's (Rockville, MD, www.fda.gov) first approval in the United States of a vaccine for humans against the H5N1 influenza virus marks an important step forward in protecting the public against a pandemic influenza outbreak.

Intranasal Therapeutics, Inc. (ITI, Montvale, NJ, www.intranasal.com), has appointed Peter F. Young as president and chief executive officer.

Neotropix, Inc. (Malvern, PA, www.neotropix.com), a biotechnology company dedicated to the development and commercialization of virus-based therapeutics for the treatment of cancer and other diseases, received a warning letter (http://www.fda.gov/foi/warning_letters/b6308d.pdf) on March 23, 2007, citing deviations from good laboratory practices (GLP) regulations governing the proper conduct of nonclinical studies as published under 21 CFR Part 58.

Cellexus Biosystems (Cambridgeshire, UK, www.cellexusbiosystems.com) has selected Julie Bick, MD, as its chief scientific officer.

The FDA has licensed Baxter Healthcare's (Deerfield, IL, www.baxter.com) Ceprotin, the first biologic treatment for patients with a rare genetic defect that can cause a potentially life-threatening clotting disorder.

Abbott (Abbott Park, IL, www.abbott.com) recently announced the official opening of its new biologics manufacturing facility in Puerto Rico to support the long-term supply of its leading biologic agent, HUMIRA (adalimumab), and other future biologics.

Singapore's efforts to grow its biologics manufacturing sector received a significant boost on March 28, 2007, when the Singapore Economic Development Board (EDB) announced that Genentech, Inc. (San Francisco, CA, www.gene.com) has decided to establish a commercial-scale microbial-based biologics manufacturing facility in Singapore.

Joanne Less has become the acting director of the US Food and Drug Administration's Office of Combination Products (OCP).
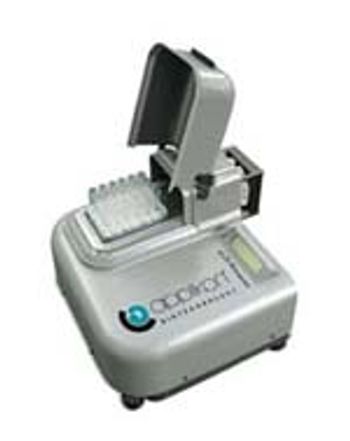
The µ24 bioreactor from Applikon (www.applikon.com) offers a platform for fermentation in a very small volume.

Patients receiving palliative care in hospices, hospitals, and other settings can benefit from a subcutaneous injection of morphine with Hylenex recombinant (hyaluronidase human injection).

New Brunswick Scientific (www.nbsc.com) has introduced a compact benchtop cell culture bioreactor capable of regulating 32 process loops per vessel, and up to four vessels simultaneously, including integrated control of multiple sensors, scales, analyzers or other external devices.

Nautilus Biotech (Evry, France, www.nautilusbiotech.com) has signed a collaboration and license agreement with HanAll Pharmaceutical (Seoul, South Korea, www.hanall.co.kr) to develop and commercialize three Nautilus Biotech products in South Korea: Belerofon (Interferon alpha); Vitatropin (human growth hormone); and EporalTM (erythropoietin).

Timothy Stitely will become FDA's chief information officer (CIO) effective March 5, 2007.

Following a series of recent setbacks, Acambis plc (Cambridge, UK, www.acambis.com) has initiated a restructuring, aimed at increasing the focus of its resources on key programs and core operational capabilities, and significantly lowering its cost base.

An FDA advisory panel has recommended that the agency approve Sanofi-Pasteur's (Lyon, France, www.sanofipasteur.com) H5N1 bird flu vaccine.