
Recently published research demonstrates how nanoparticles can be used to overcome hurdles in localized drug delivery.
Scientific Editor, BioPharm International

Recently published research demonstrates how nanoparticles can be used to overcome hurdles in localized drug delivery.

Glenn Thorpe of West Pharmaceutical Services gives an update on packaging and delivery methods for vaccines.

Marco Chacon of Paragon Bioservices discusses the challenges associated with outsourced vaccine manufacturing.

The President’s Council of Advisors on Science and Technology (PCAST) released a report containing recommendations for boosting innovation in drug discovery and development.

ISPE has published a new guidance titled ISPE Good Practice Guide: Quality Laboratory Facilities, which defines design guidelines for quality laboratories.

NIH has awarded ten laboratories two-year grants to develop tissue chip technology, with part of the funding coming from the recently established National Center for Advancing Translational Science.

Howard Levine of BioProcess Technology Consultants talks about what industry needs to know to enter the biosimilars game in the US.

Pfizer and Mylan have agreed to establish an exclusive long-term collaboration to develop, manufacture, distribute, and market generic drugs in Japan. The products included in the collaboration are expected to be sold under the Pfizer brand with joint labeling.

South Carolina-based Altec Medical pleaded guilty to one count of conspiring to defraud FDA and to commit federal offenses in connection with a drug-diversion scheme that lasted from 2007 to 2009.

Elan Corporation announced plans to spin off its discovery unit and Neotope Biosciences division to create an independent entity focused on R&D.

Gilead Sciences announced that it is entering into agreements with Indian generic drug manufacturers to enhance access in developing countries to its anti-HIV medicine, emtricitabine.

A team from Northwestern University has demonstrated the feasibility of topical delivery of small interfering RNA (siRNA).

First start-up funded by Merck Serono's Entrepreneur Partnership Program announced.

Sandoz announced it has completed the acquisition of Fougera Pharmaceuticals, a maker of generic dermatology products, for $1.5 billion.

EMA’s Committee for Medicinal Products for Human Use (CHMP) has recommended the approval of Glybera (alipogene tiparvovec, marking the first recommendation in Europe for a gene therapy medicine.

Par Pharmaceuticals, a maker of generic drugs, has entered into an agreement to be acquired by the private investment firm, TPG.

FDA approved a risk evaluation and mitigation strategy for extended-release and long-acting opioid medications.

GlaxoSmithKline has agreed to plead guilty and pay $3 billion to resolve criminal and civil liability resulting from unlawful promotion of certain drugs, failure to report safety data, and alleged false price reporting practices.
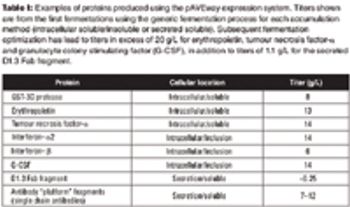
Industry experts discuss methods for optimizing protein expression in bacterial and mammalian cell lines.

In a 5-4 ruling, the US Supreme Court upheld the provision of the Affordable Care Act requiring all adults to purchase health insurance or pay a penalty-the so-called individual mandate.

Roche announced that it will be closing its R&D site in Nutley, NJ, eliminating approximately 1000 positions.

Eli Lilly announced an increase in its manufacturing network in China through an expanded collaboration with Novast Laboratories, a generic and specialty pharmaceutical company based in Nantong, China.

Takeda Pharmaceuticals announced that it has completed the acquisition of URL Pharma for an upfront payment of $800 million.

Panayiotis P. Constantinides of Biopharmaceutical & Drug Delivery Consulting on growth of nanoparticle delivery systems.

Charles H. Squires of Pfenex discusses advances in expression platform solutions.

Federal marshals seized an unapproved topical corticosteroid medication from California-based Crescendo Therapeutics.

Osiris Therapeutics announced that it received marketing authorization from Health Canada to market its stem cell therapy, Prochymal, for the treatment of graft-versus-host disease in children.

Senators Max Baucus (D-Mont.) and Chuck Grassley (R-Iowa) send letters to opoid manufacturers and pain groups asking them to disclose financial ties.

At the request of FDA, the IOM prepared a report containing recommendations for how FDA might better monitor the safety of drugs after they have been approved.

A Q&A with Alan Shaw of Vaxinnate. This article is part of a special section on expression systems.