
The agency started the full operation of its medical literature monitoring on Sept. 1, 2015.

The agency started the full operation of its medical literature monitoring on Sept. 1, 2015.

The company is in discussions with Europe’s Adaptive Pathways Group on clinical trials that would evaluate its PLX cells for the treatment of specific diseases.

European Union regulators said Pfizer must divest its infliximab biosimilar candidate and some sterile injectables for the merger with Hospira to be completely approved.

EMA’s revised guideline on the implementation of accelerated assessment is open for public consultation.

The EMA, FDA, and EC met to plan collaboration on pharmaceutical drug development and evaluation.

The European Medicines Agency reviews the safety of human papillomavirus vaccines.

The European Union has a challenging task ahead as it strives to harmonize regulations on advanced therapy medicinal products.

The agency launches initiative to stimulate pediatric drug development.

The agency has recommended granting marketing authorization for Opdivo.

The European Medicines Agency releases findings from marketing authorization application analysis.

The European Medicines Agency releases guidelines for addressing and reporting risks associated with medication errors.

Early access to Merck’s Keytruda in the United Kingdom is granted under the UK’s Early Access to Medicines Scheme.

The agency is enlisting members of European pharmaceutical associations and industry representatives to participate in IDMP task force.
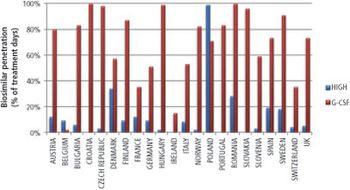
Investors are lining up for the biosimilars market as patents reach expiration and regulatory pathways are defined.

EMA is under pressure to exert even tighter standards on biosimilars being marketed in Europe.

The agency creates an electronic central repository to facilitate drug safety assessment reports.

New drugs submitted for approval in Europe have 18 months to comply with new elemental impurities guidelines.

The European Medicines Agency has published a guide to help industry and regulatory authorities implement safety-monitoring standards.

The European Medicines Agency plans on sharing generic-drug assessment reports with regulators outside the European Union.

The European Commission’s new structure has sparked controversy about its allocation of responsibilities and the impact on the development and approval of new medicines.

The first stem-cell medication approved in the EU promotes regeneration and healing to the outer layer of the cornea.

Medtronic announced that it received clearance from both the FTC and European Commission to acquire Covidien for $43 billion.

The European Medicines Agency responds to the European Ombudsman's letter regarding redacted documents.

The authors take a look at some of the recent developments in the German pharmaceutical market.

The final guidance explains some principles for developing biosimilars and establishes some rules about extrapolation across indications for various medical conditions.