
Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

Pharmaceutical Technology and BioPharm International will present a Keynote Session on Meeting Bioprocessing Manufacturing Capacity Demands on Wednesday, April 3, 2019, during INTERPHEX 2019 at the Javits Center in New York City.

A $100-million investment will expand a range of the CDMO’s service capabilities and offerings.

The company is set to expand biologics and fill/finish capacity at its biologics manufacturing sites in Madison, WI, and Bloomington, IN.

The Massachusetts site, formerly associated with an affiliate of Shire, is Rentschler Biopharma’s first facility in the US.

Avara Pharmaceutical Services acquired Sandoz’s sterile manufacturing facility for injectable medicines in Boucherville, Quebec, Canada.

More than 120 healthcare organizations plan to bring competition to generic drug market.

The acquisition is expected to support Emergent BioSolutions’ focus on public health threats and emerging infectious disease.

This article highlights 15 years of changes in biopharmaceutical manufacturing.

Rentschler Fill Solutions and Ultragenyx announce fill and finish agreement for the US commercial supply of Mepsevii.

In adding a Vanrx Pharmasystems aseptic filling isolator, FUJIFILM adds fill/finish for gene therapies and viral vaccines.

The contract manufacturing organization’s facility in Boulder, CO, has passed general inspection from FDA.
Developments and investments in single-use systems advance upstream biomanufacturing.

Grand River Aseptic Manufacturing announces first planned investment in capacity expansion.

The companies have been awarded a collaborative grant of £1.9 million (US$2.6 million) from Innovate UK.

Paragon Bioservices will build a new cGMP facility in Maryland and expand its existing cGMP facility at the University of Maryland's BioPark.

The contract research, development, and manufacturing organization has expanded API aseptic manufacturing capacity at its Valladolid, Spain, facility.

A new facility type integrates next-generation mobile cleanroom systems.

Groninger will showcase its FlexPro 50 line for small batch production at CPhI Worldwide 2017, and present on up- and downstream processes with freeze-dryer developer and manufacturer Martin Christ.

Fresenius Kabi broke ground on a previously announced $250 million expansion of its Melrose Park, IL, manufacturing facility.

Fagron Sterile Services has voluntarily recalled three lots of Succinylcholine Chloride 20mg/mL 5mL syringe to the hospital/clinic level.

Operated by BioOutsource, Sartorius’ subsidiary, the Glasgow, UK-based service center will offer physicochemical properties and structural attributes testing and allow clients to perform structural and functional analyses in parallel.

Modular Automated Sampling Technology (MAST) allows direct aseptic transfer of bioreactor samples to analytical devices, providing rapid and reliable data in bioprocessing.
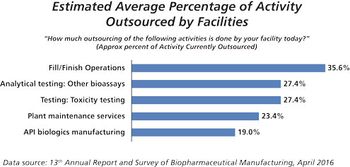
This key bioprocessing segment is expecting continued growth.

Baxter expands capacity for lyophilized cytotoxic oncology therapies at its fill/finish facility in Halle, Germany.

The European Medical Contract Manufacturing (EMCM) organization announced it will team up with VCC Medical NV for a unique personalized medicine initiative-the aseptic manufacture of a biologic therapeutic from the tissue of a patient’s tumor.