
CMO's Ratchet up Use of Single-Use Devices

CMO's Ratchet up Use of Single-Use Devices

Clamor mounts over compromised care and rising costs due to lack of crucial therapies.
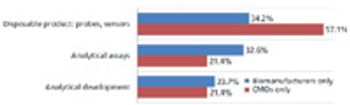
Biosimilar manufacturers need better expression systems and analytical tools to compete.

Industrializing design, development, and manufacturing of therapeutic proteins.

With the rise in therapeutics comes more complex partnerships.

At a time when the industry is struggling with innovation, it might do well to learn a lesson from a few great innovators.
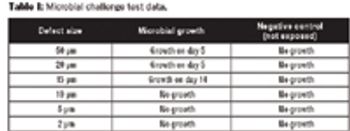
Defects as small as 10 μm can be detected without compromising product cleanliness using helium integrity testing.

In this quarter's column, highlights from the IBC Single-use Applications meeting, the PDA Single-use Workshop, and the BioProcess systems Alliance International Single-Use Summit are presented.

Single-use systems continue to gain traction among biomanufacturers, especially CMOs.
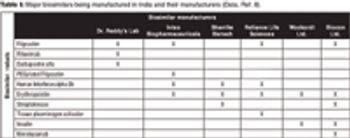
The author explains the current status of India, the challenges, and recommendations that may alleviate these challenges.
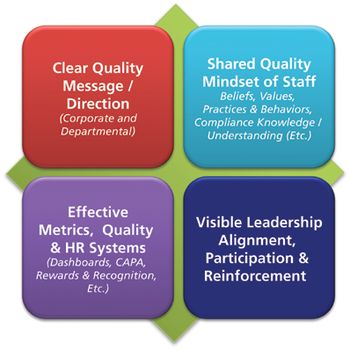
In a culture of quality, it is important that employees adopt this mindset, not because they have to, but because they understand the importance.
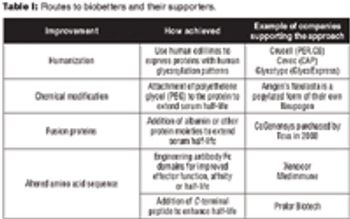
Despite their difficult approval pathway, biobetters offer the potential for innovation and decreased healthcare costs.