
How to Put QbD into Practice

How to Put QbD into Practice

What football and bioprocessing both have in common is that in both cases, success is a minimum requirement.

How to reduce plasmid-mediated metabolic burden for higher yields.

An enterprise-wide quality management initiative is required to maintain supplier quality without sacrificing bottom-line objectives.
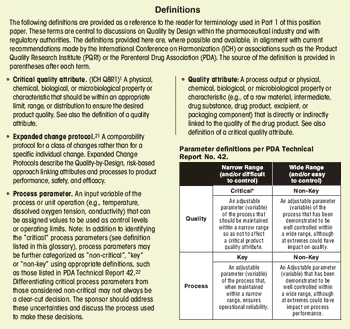
First in a three-part series that discusses the complexities of QbD implementation in biotech product development.

The focus on the design space will lead to a new workspace, and will affect staff in the development, manufacturing, and quality functions.
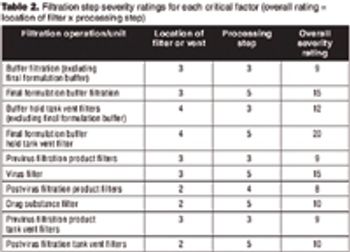
Conducting a FMEA analysis is a good first step in a risk-based approach for determining the need for a filter integrity test.

Heightened attention to product safety issues is slowing the approval process for new therapies.