
Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.

Manufacturers work with international authorities to harmonize drug registration and supply-chain oversight.
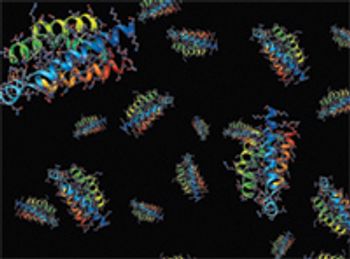
The author describes a method to avoid protein aggregation when using light scattering systems.

While there are those who want combination products to be controlled by a centralized pharmaceutical-type approval system, the majority of the medical technology industry wants to retain a decentralized device-focused approach.

KR Karu from Sparta Systems spoke with BioPharm International about the importance of having an enterprise quality management system.

Prior to price escalation of pharmaceutical products in Brazil, the country's regulatory authority released a study on price-cap control and its benefits in the past years.

The author discusses issues related to the supply of soft parts in the biopharma industry.

The author presents best practices for extractables and leachables.

BioPharm International spoke with INTERPHEX 2013 conference-session presenters to gain insight on trends in facility and process design.

Additional challenges to the new cleanroom paradigm from concurrent multiproduct manufacturing of bulk drug substances in a controlled non-classified (CNC) ballroom environment.
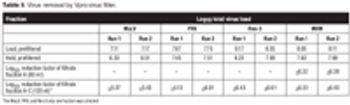
This study on a recombinant human follicle stimulating hormone demonstrates the use of virus filters to reduce the risk of contamination.

Wanted: Aspiring authors to share technical and scientific solutions for biopharmaceutical processing.

Outsourcing is weighing in more as a tactic for cost-cutting, but it is still not the primary weapon.
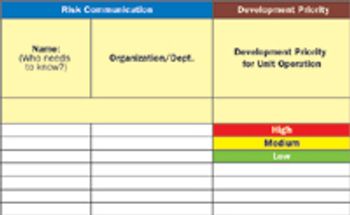
Quality risk management is an essential element of every aspect of drug development and manufacturing throughout the product lifecycle.

Click the title above to open the BioPharm International May 2013 issue in an interactive PDF format.

Monoclonal Antibodies : Developing an Alternative Polishing Step