
Manufacturers face demands for timely information on clinical studies, product recalls, and approvals.

Manufacturers face demands for timely information on clinical studies, product recalls, and approvals.
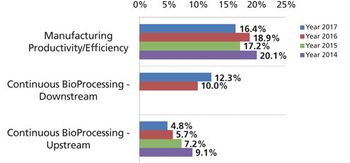
Development and adoption of new technologies create challenges that may take years to resolve.
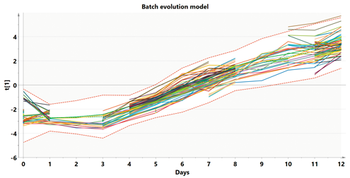
Modeling at various stages of the data analytics continuum aids scale comparison of a bioreactor.

In-house experts can help select the right systems and suppliers, making validation and compliance easy, says Siegfried Schmitt, principal consultant at PAREXEL.

FDA enforcement efforts and drug approvals trend upward.

Lower costs, fewer opportunities for temperature excursions, and a smaller carbon footprint are making ocean transport more attractive for pharmaceuticals. Poseidon, a new collaborative pharma initiative, seeks to leverage benefits.

Alan Kennedy, executive director of TEAM UP, shared perspectives on Poseidon and ocean transport.
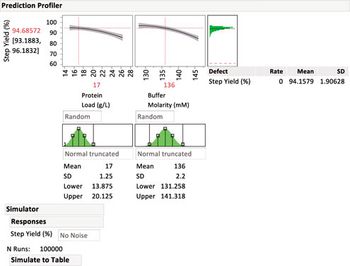
Statistical methods to identify critical process parameters and critical material attributes-and approaches to control them-are needed to protect drug product and drug substances.

Spectroscopic tools present an alternative method for reliable at-line process monitoring and control.

Biologic new molecular entities (NMEs) accounted for 26% of total NME approvals in 2017.

Click the title above to open the BioPharm International March 2018 issue in an interactive PDF format.