
The supply base for preclinical and clinical development services continues to expand in China.

The supply base for preclinical and clinical development services continues to expand in China.
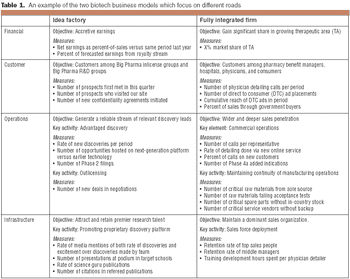
Two business models that companies can follow to map their strategy from concept to implementation.

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.

More informed submissions may lead to regulatory flexibility for postapproval changes.
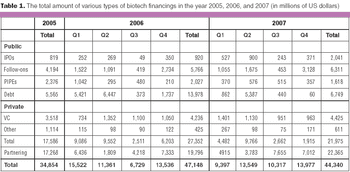
Despite the rising fears about a slowing economy, the biotech industry will continue to maintain its momentum this year.
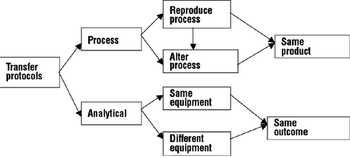
A comprehensive process and analytical transfer package can speed up your product's time to market and save costs.

How an electronics engineer led the first Indian company to carry out indigenous development of a recombinant vaccine.
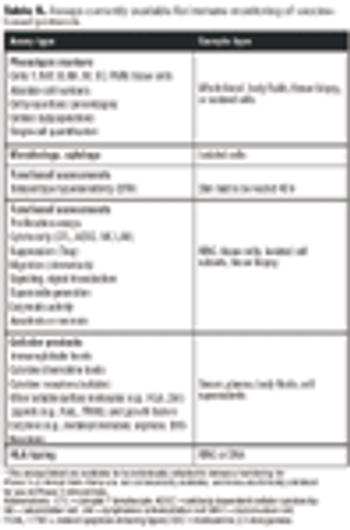
Emerging therapies pose challenges for standardizing QC.

The expanding globalization of the industry poses a challenge for international enforcement.

The cause of the heparin crisis is still unknown. We do know, however, that the FDA is severely underfunded.

Need a standard to follow? Just ask, What would Genentech do?