
Viral Vaccines

Viral Vaccines

Development requirements and regulatory guidance for biosimilars and biobetters.

NIBRT's Michael Lacey provides an overview of biopharmaceutical facility design and operation.

Ever since Twitter launched in 2006 and Facebook became mainstream, most industries have sought ways to connect with their consumers through social media.

Shortages spur efforts to overhaul manufacturing oversight.
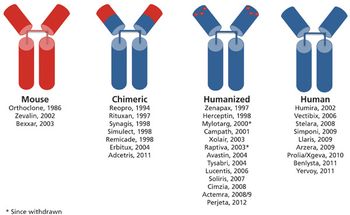
Innovative products and a range of indications drive the therapeutic antibody market.

A look at vaccine history, markets, manufacturing, and overcoming the scale-up dilemma.

Project: transfer a manual concentration/diafiltration process for siRNA production.

Is the contract-only CMO an endangered species?

Tony Hitchcock of Cobra Biologics discusses challenges posed by production of viral vectors for vaccines.

Eastern Europe is moving towards a goal of harmonized regulations.

India's biopharmaceutical industry is valued at $2 billion a year with 20 companies producing biosimilars and 50 products available in the domestic market.

Improvement strategy should be linked to business strategy.