
The agency will review Praluent (alirocumab) Injection, a PCSK9 inhibitor, as a possible treatment of cardiovascular events.

The agency will review Praluent (alirocumab) Injection, a PCSK9 inhibitor, as a possible treatment of cardiovascular events.

The contract development and manufacturing organization expanded its analytical chemistry suite and added a new office in Boston, MA.

The company will be presenting at CPhI Worldwide on Oct. 11, 2018.

BeiGene is set to build late-stage clinical and commercial production capacity for cancer monoclonal antibodies with GE Healthcare’s KUBio, the prefabricated biopharma facility based on single-use technologies.

The biopharmaceutical company will invest approximately $800 million to expand facilities and manufacturing capacity at its campus in Rensselaer County, NY.
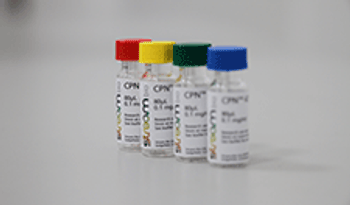
The companies have partnered to launch Conjugated Polymer Nanoparticle (CPN) products for use in molecular imaging and R&D applications.

The drug particle engineering and nanotechnology company offers a nanotechnology platform that can revive failed drugs in the pharma pipeline.

PCI Pharma Services and CSP Technologies will partner on protective packaging solutions for clinical trials and stability testing.

Colin Clarke, from the National Institute for Bioprocessing Research and Training, will lead a four-year European Industrial Doctoral Program for enhancing upstream biopharmaceutical manufacturing process development through single cell analysis.

FDA sent a warning letter to Lernapharm (Loris) Inc. detailing the company’s lack of procedures to prevent microbiological contamination.

In the second half of CPhI’s annual report, experts review industry trends and warn that trade and patent changes could increase healthcare cost by $100 billion over the next five years.

The provider of plant-based ingredients will present recently launched multi-compendial materials for upstream and downstream biopharmaceutical applications.

Researchers at Vanderbilt University Medical Center have isolated the first human monoclonal antibodies (mAbs) that can neutralize norovirus, a virus that causes acute gastrointestinal (GI) illness.

Researchers from Ruhr-Universität Bochum in Germany and the National Institutes of Health modified the protein Nurr1 to enter cells from the outside, possibly enabling the protein to become a drug development candidate for illnesses such as Parkinson’s disease.

The companies will develop therapies targeting the in-vivo elimination of hepatitis B virus (HBV) with Precision’s proprietary genome editing platform.

Avara Pharmaceutical Services acquired Sandoz’s sterile manufacturing facility for injectable medicines in Boucherville, Quebec, Canada.

FDA testing has found an additional impurity, N-Nitrosodiethylamine, in the API valsartan.

The agency approved AstraZeneca’s Lumoxiti (moxetumomab pasudotox-tdfk) injection for intravenous use for the treatment of adult patients with relapsed or refractory hairy cell leukemia.

The new features on the purifier deliver additional sizes and sterilization/sanitization compatibility.

The collaboration will focus on developing manufacturing solutions for biosimilars.

Gore’s new flexible freeze containers are designed to protect high-value drug substances from container breakage or leakage.

The collaboration will explore the potential of Dyadic’s gene-expression platform to produce multiple biologic vaccines and drugs.

Bio-Rad introduces CHT Ceramic Hydroxyapatite XT media and Nuvia HP-Q resin resin for process protein purification.

New products were developed as next-generation process intensification technologies, MilliporeSigma reports.

ICS distribution center will serve manufacturers of specialty medications, biosimilars, and cell and gene therapies.

Becton Dickinson’s (BD) Advanced Bioprocessing business will be integrated into Thermo Fisher's Life Sciences Solutions segment.

Research from the Perelman School of Medicine at the University of Pennsylvania suggests that a universal flu vaccine that protects people against most influenza strains is one step closer to reality.

Emergent BioSolutions is set to acquire Adapt Pharma, a pharmaceutical company focused on addressing the opioid overdose and addiction crisis, in a deal worth up to $735 million.

Novartis will sell selected portions of its Sandoz United States portfolio to Aurobindo Pharma USA for $900 million in cash, plus $100 million in potential earn-outs.

Lonza’s new PyroTec Pro Robotic Solution provides a fully automated workflow for endotoxin detection.