
The 135,000 square foot facility will be constructed over 18 months and is expected to be operational in 2021.

The 135,000 square foot facility will be constructed over 18 months and is expected to be operational in 2021.

At the event, Centogene will also announce its plans to host the annual International Rare Disease Film Festival in Berlin in 2021.

FDA will observe Rare Disease Day on Feb. 29, 2020, with events surrounding the day’s 2020 theme, “Reframe Rare for Rare Disease Day.”

WuXi Vaccines, WuXi Biologics’ joint venture with Shanghai Hile Bio-technology, has signed a 20-year vaccine manufacturing contract with a global vaccine leader for $3 billion.

Sanofi will use a recombinant DNA platform that produces a genetic match to proteins found on the surface of COVID-19 to formulate the vaccine.

An evaluation by USP indicates bovine heparin is a potential alternative to porcine heparin.

FDA’s Center for Biologics Evaluation and Research is planning on publishing nine specific guidance documents on gene therapies in 2020.

Ongoing coronavirus outbreak prompts date change to ensure safety of trade show participants.

The agency is taking steps to monitor the supply chain and assist in the development of treatments.

The company has said that all three of its operating sites in China started back up on Feb. 12 and that it is closely monitoring the outbreak.

Under the agreement, Roche will have access to Promedior's lead product candidate, PRM-15, a recombinant form of human pentraxin-2 that can possibly treat a range of systemic fibrotic diseases.

The companies have entered into a global licensing and collaboration agreement to commercialize ReForm excipients used in biotherapeutic formulations.

If approved, this treatment will be the first therapy targeted for METex14-mutated advanced lung cancer.

ERS Genomics revealed that the European Patent Office (EPO) has rejected arguments filed in opposition to patent EP2800811, which is directed to the single-guide CRISPR/Cas9 gene editing system and covers uses in cellular and non-cellular settings.

The Native Antigen Company has commercially introduced antigens that have been specifically derived from the Wuhan strain of novel coronavirus, now named Covid-19.

The report details OPQ’s accomplishments over the past five years.

Through the agreement, Catalent will offer process optimization and drug substance manufacturing services for the drug candidate at its Madison, WI site.

Jim Walter will take on the role of vice-president of operations for Catalent’s Oral and Specialty Delivery business.
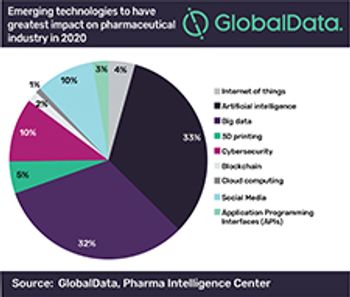
A growing number of pharma executives see investment in machine learning and big data as a top priority, according to a 2020 GlobalData survey.

FDA published draft guidance for applicants seeking licensure of a proposed biosimilar or proposed interchangeable biosimilar.

The two agencies are collaborating to support a robust biologics marketplace by taking steps to deter anti-competitive business practices.

The new facility includes six classified environment rooms with space to expand.

Aimmune plans to introduce the antibody as an adjunctive treatment with its Characterized Oral Desensitized ImmunoTherapy programs to research treatment outcomes in patients with food allergies.

Catalent builds on its investment in cell and gene therapy development and manufacturing with the acquisition of MaSTherCell Global.

The knockout CHO K1 cell line will be used to support biotherapeutic R&D across a range of therapeutic indications.

The vaccine is designed to provide active immunity against the influenza A (H5N1) strain and can be easily deployed in a pandemic event.

The UK BioIndustry Association (BIA) has issued a statement welcoming the publication of the Life Sciences 2030 Skills Strategy, a report setting out how the life sciences sector in the United Kingdom will develop future talent.

Abcam has purchased Applied StemCell’s (ASC’s) gene editing platform and oncology product portfolio, adding comprehensive cell editing capabilities and engine to support expansion of existing “off-the-shelf” cell lines.

The partners will collaborate on developing scale-up chip-based technology to enable commercial-scale production of a third-generation DNA synthesis platform.

The company’s new Milliflex Oasis System provides enhanced result reliability, increased productivity, and advanced traceability.