
After a series of government reforms, the Japanese pharma market is making a comeback.

After a series of government reforms, the Japanese pharma market is making a comeback.

Introducing a new way to think about sharing information in a patent-driven industry.

Did the 2005 Patents Act engender a Western intellectual property rights culture in the country?

India is restructuring its regulation of biopharmaceuticals to help the country's industry compete internationally.
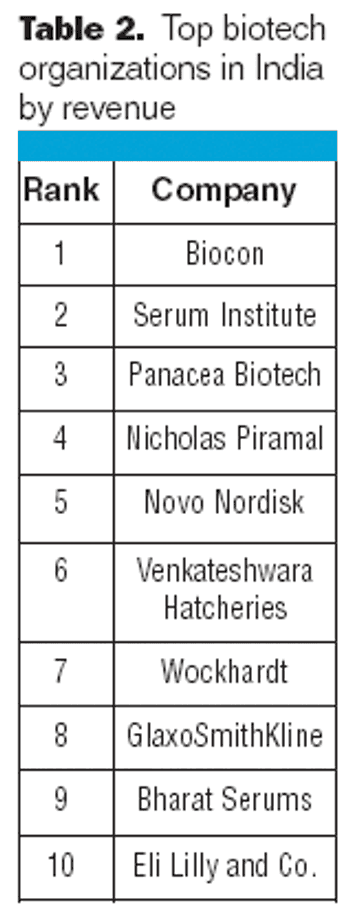
Indian biogenerics could form a major piece of the global biotherapeutics market in the future.

Many companies acknowledged that Western regulatory standards are tougher than those in China.
The corruption of China's food and drug sectors is not limited to one man. Likewise, real reform will require the efforts of many.
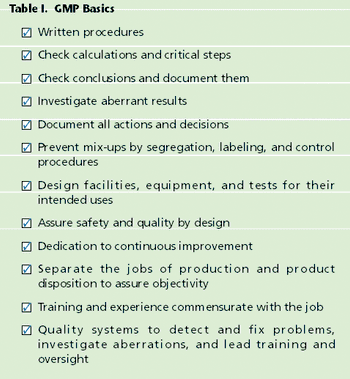
What are current good manufacturing practices (cGMPs)? Where did they come from? What are the actual "practices" described in the Code of Federal Regulations, 21 CFR. If you are new to the pharmaceutical or biotechnology industries, you may enter your first "GMP Training" session without much context or perspective. A set of arcane rules is presented; you were never taught these in science classes.