
A guidance document published just before the Jan. 1, 2015 deadline adds a four-month grace period.

A guidance document published just before the Jan. 1, 2015 deadline adds a four-month grace period.

A statement from GPhA’s president expresses the organization’s support of FDA’s proposed rule to amend labeling regulations.

Manufacturers face regulatory overhaul, while brand-generic debates escalate over biosimilars and labeling changes.

FDA clarifies recommendations for injectable drug products packaged in vials and ampules.

Manufacturers are taking measures to comply with new package safety rules.

A packaging defect has led to Merck voluntarily recalling all lots of Liptruzet tablets in the United States.

BioPharm International speaks with industry experts about challenges faced in managing the cold chain.

USP is developing and revising distribution standards in response to changes in the global supply chain.

The authors discuss how to develop a cost-effective thermally protective packaging system.

FDA Issues Draft Guidance on Patient Counseling Info for Labeling
A Q&A with SCHOTT Pharmaceutical Systems and West Pharmaceutical Services

The European Commission (EC) is seeking to introduce a black symbol to identify medicines that are subject to additional monitoring.

New US Pharmacopeial Convention (USP) standards provide a universal approach to organizing labels for prescription containers dispensed by US pharmacists in an effort to improve patient understanding.
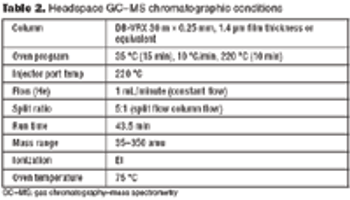
A systematic approach facilitates formulation component selection.

Formulations for pulmonary inhalation comprise spherical, porous particles that are 1–3 microns in diameter.

On August 12, 2003, Johnson & Johnson began recalling certain batches of its anemia drug, Eprex (epoetin alfa, sold as Procrit in the US), in most countries outside of the United States.