
This approval marks the second gene therapy to be approved by FDA and the first to be approved for certain types of non-Hodgkin lymphoma.

This approval marks the second gene therapy to be approved by FDA and the first to be approved for certain types of non-Hodgkin lymphoma.

Baxter and FDA are working together to prevent shortages of the company’s sodium chloride 0.9% injection bags after recent hurricanes damaged the island.

AbbVie, Amgen, AstraZeneca, Bristol-Myerse Squibb, and Eli Lilly and Company said they are working to restore normal operations at their respective facilities and continuing recovery efforts.

The acquisition adds to Catalent’s capabilities in biologics development, analytical services, manufacturing, and finished product supply.

Alexion’s restructuring will reduce its global workforce by 20%, including closing a manufacturing facility in Rhode Island.

FDA approves Novartis’ CAR-T therapy, marking the first time a cell therapy based on gene transfer has been approved in the United States for any indication.

The Senate voted 94–1 to approve legislation reauthorizing user fees and a series of program changes.

A Merck, Pfizer, and Corning collaboration resulted in development of Corning Valor Glass for improved drug storage and delivery and will create US jobs.

After FDA found a “significant shift in the route of abuse,” Endo voluntarily removes OPANA ER from the market.

The agency announced a plan to eliminate its existing orphan designation request backlog.

The landmark decision determined that biosimilar makers can notify manufacturers before receiving FDA approval.

FDA asked Endo Pharmaceuticals to remove Opana ER from the market, citing the potential for abuse.

Thermo Fisher will acquire Patheon for approximately $7.2 billion, including the assumption of approximately $2 billion of net debt.

User fee reauthorizations, a hiring freeze, and opioid epidemic await the new FDA commissioner Scott Gottlieb.

The program aims to make biosimilars for the treatment of cancer more widely available in middle- and low-income countries.

Forecasts were lowered, Reuters reported, due to reduced drug approvals and increased competitive pressures.

The agency approved Renflexis, a biosimilar to Janssen’s blockbuster rheumatoid arthritis treatment.

The dispute over who has the rights to key CRISPR-Cas9 patents continues. On April 12, 2017 the University of California, Berkeley, in conjunction with the University of Vienna and Emmanuelle Charpentier, filed an appeal to overturn an earlier decision by the United States Patent Trial and Appeal Board (PTAB).

The mAb is the first approved treatment that targets the progressive form of the disease.

Human antibody for Zika virus could help in the treatment and prevention of the infection.

Trump’s choice for FDA commissioner faces drug pricing, regulatory, and approval challenges.

The White House said President Trump will nominate Scott Gottlieb to the position of FDA commissioner.

The Mutual Recognition Agreement will allow FDA and EU inspectors to recognize each other’s work and avoid the duplication of drug inspections.

PhRMA submits comments to the The Office of the United States Trade Representative encouraging protection of US innovation in foreign markets.

The Patent Trial and Appeal Board ruled in favor of the Broad Institute, allowing the company to keep patents for their CRISPR-Cas9 gene-editing technology.
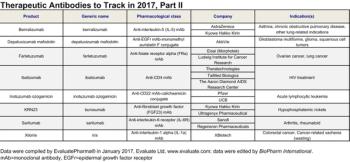
Evaluate and BioPharm International highlight the antibody-based therapeutics that may have 2017 launch dates in the United States.

Drug companies are pushing Congress to action on funding for FDA programs and staff to expedite drug reviews and approvals.

EvaluatePharma and BioPharm International highlight the antibody-based therapeutics that may gain United States Regulatory approval in 2017.

A total of 166 biotech executives penned an open letter expressing concern over President Donald Trump’s executive order on immigration.

Aggressive petitioning by ViroPharma kept a generic equivalent to Vancocin off the market for more than two years.