
Recognize that the drug drought is the "disease" underlying many of pharma's troubling symptoms, and the cure becomes apparent.

Recognize that the drug drought is the "disease" underlying many of pharma's troubling symptoms, and the cure becomes apparent.

Big Pharma execs bring to biotech the knowledge of what it takes to make something a commercial success-not getting to market, but to Phase I.

EVERYONE KNOWS THAT ROCHE WAS, AND ARGUABLY STILL is, the driver of Genentech's success. But not everyone knows that Roche owns a majority stake in the biotech behemoth, as it does in Japanese powerhouse Chugai. And that's by design. Roche's management has long held that the best way to derive good and lasting results from its smaller partners is to let them do what they do best and leave them alone while they do it. That philosophy has served them well, especially when revenues of some of their codeveloped and comarketed blockbuster products are considered. Just two, Genentech's Herceptin (trastuzumab) and Chugai's Epogin (epoetin beta), have earned billions.
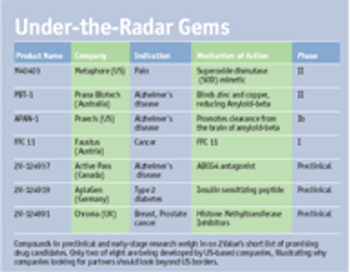
Companies should dig in a more attractive mine where very few other companies are looking.
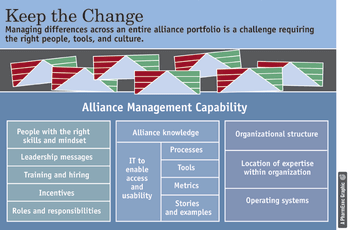
He potential for frustration, time-wasting, and bad feelings between pharma and biotech partners is enormous. That some successful decisions are made under these circumstances is something of a miracle.
More than half the deals made by pharma are with companies based in the US, 28 percent in Europe, and 15 percent Japan.

The "due diligence framework" is an efficient process. But efficient is not the same as quick and easy. There are no shortcuts. The framework minimizes resource commitments and disruptions to ongoing programs and helps each side learn what it needs to know. In six steps, it converts the typical due diligence root canal into a painless filling.