
Representatives from leading CROs weigh in on key challenges tied to the biopharmaceutical R&D, including issues regarding bioequivalence, platform technologies, process analytics, and more.

Representatives from leading CROs weigh in on key challenges tied to the biopharmaceutical R&D, including issues regarding bioequivalence, platform technologies, process analytics, and more.

The author describes an equation that can be used to define the Quality relationship between a contract manufacturing organization and a client, including how to factor in both party's needs and regulatory commitments.
An architectural wonder teaches the biopharmaceutical industry a valuable lesson in project management.
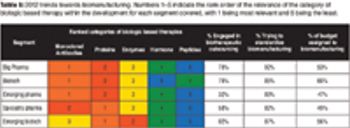
Growth in infrastructure and process-development capabilities will be required to support the biologics market.

Outsourcing in the pharmaceutical industry, until recently, has been largely confined to commercial manufacturing, packaging, and support for clinical trials.
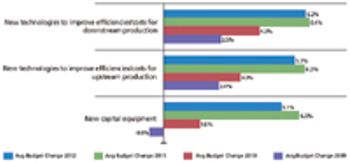
New outsourcing industry sectors have become multibillion-dollar industries.

Challenges of vaccine development include regulatory, technical, and manufacturing hurdles in translating a vaccine candidate into a commercial product.

Because of the complexity of the manufacturing processes for biologics, transfer of these processes to a contract manufacturer presents challenges.