Manufacturers introduce innovations in glass and plastic packaging for injectables.

Manufacturers introduce innovations in glass and plastic packaging for injectables.

Reeling from financial and tropical storms, Puerto Rico needs stable industry to aid its recovery.
Detecting viral contaminants in biologic-based medicines-and identifying their source-requires a holistic testing approach.

There is a lot of interest in delivering biologics via non-invasive routes in attempt to improve patient compliance and convenience.

Industry and FDA face new fee structures and new challenges in implementing fee initiatives.
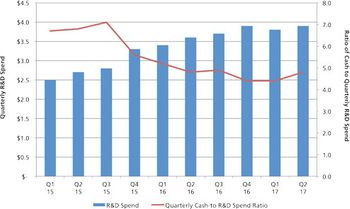
Mergers and acquisitions are positive for the CDMO industry, but there is a downside.

Focusing on whether the product meets its defined quality attributes should help one make reasonable, documentable, and defendable risk-based decisions, according to Susan Schniepp, distinguished fellow at Regulatory Compliance Associates.

Process analytical testing for biopharmaceuticals requires enhanced methods due to complex bioprocesses.
A UHPLC SEC approach for protein aggregate analysis of mAbs is presented.

This study outlines methods for an alternative protein-polishing process for challenging proteins.
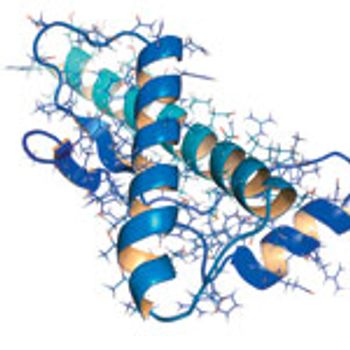
. This study is an attempt to produce a fusion protein by binding the fragment NT-gp96 in upstream of sequence of the N terminal fragment (NT300) of the NS5B gene in an expression vector.

Click the title above to open the BioPharm International October 2017 issue in an interactive PDF format.