
GMPs in Phase 1 is more important now than ever before.

GMPs in Phase 1 is more important now than ever before.
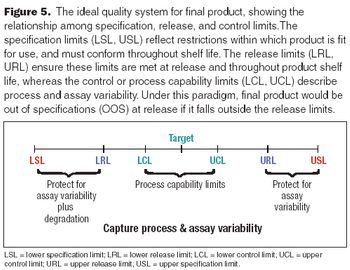
Set limits to provide incentives for process improvements.

Manufacturers of biopharmaceuticals can improve productivity by taking patient wellness into account.
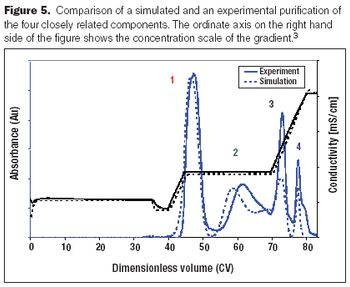
A review of some recent contributions in process chromatography.

What the Indian government is doing to make its biotech sector as strong as its IT sector.
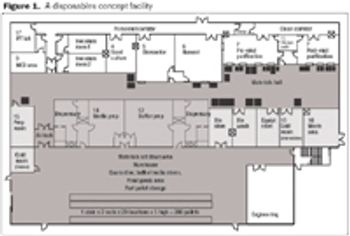
In addition to making technical developments, vendors are also looking at ways to improve supply-chain security. By offering standard, off-the-shelf products, vendors are able to shorten lead times and improve the security of supply.

The Sentinel System aims to generate more adverse event reporting by health professionals, to analyze health information more effectively, and to enhance FDA methods for communicating new safety information to providers and patients.