
TOC Analyzer Increases Productivity

TOC Analyzer Increases Productivity

Industry suppliers describe new technologies to facilitate downstream processing.

The European Pharmacopoeia Commission re-evaluates its policy on the development of monographs for finished drug products.

Regulators and industry organizations explain policies and standards to manufacturers and authorities in all regions.

Protecting intellectual property rights is vital to biopharmaceutical innovation.
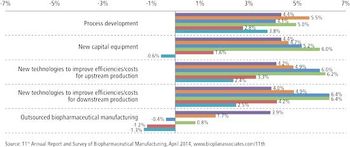
With budgets growing, clients see CMOs' costs as less crucial.

Experts give insight on method transfer, QbD, and regulations for analytical method development and validation for biopharmaceuticals.

Brunei harnesses its rich biodiversity and the growing halal market in a bid to develop its pharmaceutical sector.

New test methods can provide improved quality and efficiency, but they must be validated to demonstrate equivalency.
Handheld Analyzer Improves Accuracy

Different approaches to prepare highly concentrated feed media for fed-batch Chinese hamster ovary cell culture are evaluated.

SEC-MALS Detector for UHPLC
Suppliers see challenges to the adoption of single-use technologies for downstream processing as opportunities.

Click the title above to open the BioPharm International June 2014 issue in an interactive PDF format.