
The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.

The book is a useful, comprehensive, and truly an excellent reference source of biopharmaceutical information.

There are several steps that you can take to ensure that you get the greatest benefit from consultants.
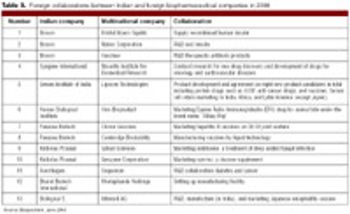
The new patent regime is challenging the Indian biopharm industry to transition from generics to novel products.

Disposable technologies that mimic the conventional stainless-steel bioreactor will be most readily adopted
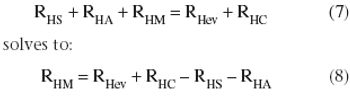
A simple method to leverage fermentation heat transfer data.

Engaging executive leadership in the quality process is the key to compliance success.

As more biotechs turn to convertible note financing instead of traditional venture capital, they need to be aware of investors' demands.

A case study investigated the root cause of failures in sterile filtration by evaluating the effects and interactions of four operating parameters.

Did the 2005 Patents Act engender a Western intellectual property rights culture in the country?

The heparin safety crisis puts a spotlight on manufacturing processes and regulatory oversight.

Now is a good time for companies to know their suppliers well.