
All contributors to the process should have a clear understanding of their capacity and see their work activities as a priority, regardless of where they fall on the critical path.

All contributors to the process should have a clear understanding of their capacity and see their work activities as a priority, regardless of where they fall on the critical path.
To shorten time to market for new therapeutic proteins, new and fast methods, such as high throughput screening, are needed to speed up downstream processing. The platform technology discussed in this article includes a structural approach that can be used as a general procedure to purify therapeutic proteins. The approach starts with ligand screening and selection-on-a-chip, with the Surface Enhanced Laser Desorption Ionization–Time of Flight (SELDI–TOF) mass spectrometer system. Next, resin screening and supplier selection are performed using robotics, followed by scouting studies under dynamic conditions to select the best resin. Finally, optimization studies of critical parameters are carried out with statistical design approaches (design of experiments). A few examples are presented to explain the platform approach for purification development in more detail.

Disposables are no longer a mistrusted new technology; they're seen as a potential solution to everyday problems.

Manufacturing recombinant proteins at industrially relevant levels requires technologies that can engineer stable, high-expressing cell lines rapidly, reproducibly, and with relative ease. Commonly used methods incorporate transfection of mammalian cell lines with plasmid DNA containing the gene of interest. Identifying stable, high-expressing transfectants is normally laborious and time consuming. To improve this process, the ACE System has been developed based on pre-engineered artificial chromosomes with multiple recombination acceptor sites. This system allows targeted integration of single or multiple gene copies and eliminates the need for random integration into native host chromosomes. To illustrate the usefulness of the ACE System in generating stable, high-expressing cell lines, we present several case studies covering CHO cell lines expressing monoclonal antibodies.

Although IP due diligence is relevant to virtually any transaction between biotech companies, a detailed investigation into IP assets is particularly critical to M&A transactions.

The three largest players have accumulated, or are in the process of accumulating, nearly a million liters of capacity between them.

The Chinese government's investments in the biopharmaceutical sector may help it become one of the leading industries in China by 2020.

An underlying theme of FDA's drug safety program is that new discoveries in biomedical science can detect risk issues earlier in clinical development.
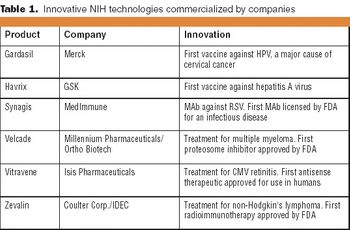
NIH makes available a full range of licenses for commercial evaluation and for the sale of commercial products and services.