
A handful of therapeutics have performed extremely well in 2012, but as a whole, life-sciences are still down from 2010.

A handful of therapeutics have performed extremely well in 2012, but as a whole, life-sciences are still down from 2010.

FDA talks about the changing scope of regulatory science.

Therapeutics targeting epigenetic mechanisms of disease will change the pharmaceutical marketplace.

Our editorial advisory board members provide their perspective on what's ahead in the biopharmaceutical industry.

Shortages spur efforts to overhaul manufacturing oversight.

The EU fine-tunes the Falsified Medicines Directive.

What the industry's future holds and what needs to be done to get there.

Brazil to locally produce HIV/AIDS antiretroviral drug atazanavir.

NIBRT's Ray O'Connor provides an overview of aseptic processing.

Neil Lewis, chief technology officer at Malvern Instruments, talks about the challenges associated with biologics.
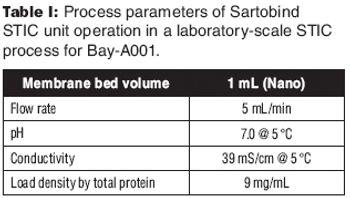
A new downstream purification platform using a salt-tolerant membrane adsorber.

A recent ISPE guidance provides a baseline for the design of quality laboratory facilities.
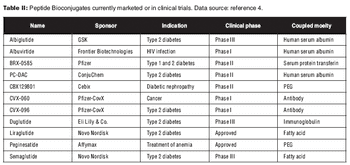
Conjugation of a biologic to a carrier molecule can solve problems in solubility and stability, but introduces its own challenges.
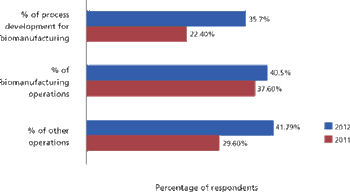
Will international biomanufacturing outsourcing become mainstream in this decade?

The Future of BioPharma Innovation