
The company’s mission to be “continuously innovative” has resulted in the creation of this novel acronym.
Patrick Lavery is an Editor for the MJH Life Sciences brands Pharmaceutical Technology and BioPharm International, and their respective websites, pharmtech.com and biopharminternational.com. Previously at MJH, he filled the same role for LCGC International and Spectroscopy.

The company’s mission to be “continuously innovative” has resulted in the creation of this novel acronym.
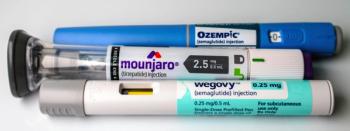
Misleading promotions of GLP-1 and compounded semaglutide products are drawing renewed regulatory scrutiny over risk disclosure and safety messaging.
A published study finds that the therapy reported specific and potent cytotoxicity against acute myeloid leukemia, T-cell acute lymphoblastic leukemia, and other rare blood cancers.
Telix will now include an additional, confirmatory efficacy study analysis of existing data, hoping to satisfy FDA’s request for supplemental evidence and address concerns raised in a complete response letter.

The agency says it will now publish complete response letters promptly, offering developers early insight into regulatory deficiencies to improve development planning.

The nonprofit is calling upon its community of experts in both artificial intelligence and machine learning to continue building support for responsible adoption of AI in the bio/pharmaceutical industry.

The partnership, which has received £118 million (US$158 million) in research funding, aims to establish a better understanding not only of how the body fights infection, but also how vaccines protect it.

Circular RNA offers enhanced stability and protein expression, making it an intriguing next-generation alternative to mRNA.

In partnership with AstraZeneca, the company previously announced approval by FDA in January 2025 for the same indications.

Using solid- or liquid-phase peptide synthesis or hybrid approaches, Snapdragon can now support peptide projects from development to GMP manufacturing.

VeonGen provided the first update to its lead investigational gene therapy since announcing a company rebrand in June 2025.

The decision was based on results of a Phase III trial that showed a median overall survival of 33.8 months with enfortumab vedotin plus pembrolizumab versus 15.9 with platinum-based chemotherapy.
Orlynvah is the first new, branded product for the treatment of uncomplicated UTIs to be introduced in the US in more than 25 years.

The agency has completed both pre-license and bioresearch monitoring inspections with no observations, and no safety-related concerns have been raised to date.

The company was one of 17 to receive letters from the White House on July 31 detailing the steps each company must take to bring prescription drug prices down for Americans.
There are no approved therapies for RRP that completely eliminate the need for repeated surgical procedures.

Type 1 diabetes is a lifelong condition that currently affects approximately 400,000 people in the UK.
The company is not developing an mRNA vaccine, but had been using one as the control in a 10,000-subject Phase IIb trial.
Surveyed by PharmTech Group, this collection of industry players offers their thoughts on HHS’ latest policy move.

While no new mRNA-based projects will be eliminated, other uses of mRNA technology within HHS are not impacted by the announcement.
The new platform delivers CAR-T DNA and transposase directly in vivo, potentially lowering manufacturing complexity and expanding treatment access.
The acquisition highlights growing investor interest in gamma delta T-cell therapies and reflects increased monetization of biotech licensing agreements.

If action is not taken within 60 days, the White House said it would “deploy every tool in our arsenal” to improve drug pricing practices for American patients.

Smart technology, personalized medicine, and the customization of CDMO relationships are helping manufacturers meet shifts in client demand.

While little information is known about this particular case, its possible implications are far-reaching.

Mirvetuximab soravtansine, brand name Elahere, is the first licensed treatment for women with platinum-resistant ovarian cancer in the UK in more than 10 years.

Following an overhaul at ACIP, the HHS secretary took the advice of the committee’s new members, saying he was acting on guidance that dated back to 1999.

Manufacturing sites and a control site will work together in a hub-and-spoke model that has key differences from conventional manufacturing operations.

Three deaths attributed to acute liver failure appear to have occurred following regular treatment or investigational therapy.
Stoboclo and Osenvelt (both denosumab-bmwo) reference Amgen’s Prolia and Xgeva, respectively.