
Top of CDER’s to-do list for 2020 is tracking adverse events more effectively and combating the opioid crisis.

Jill Wechsler is BioPharm International's Washington Editor, jillwechsler7@gmail.com.

Top of CDER’s to-do list for 2020 is tracking adverse events more effectively and combating the opioid crisis.

Can a commitment to limit prices to ensure patient access to important new medicines regain public trust and confidence in the biopharmaceutical industry?

The numbers of new molecular entities approved in 2019 are close to or exceed FDA’s performance in most previous years.

Pressures on FDA will affect industry’s success in bringing new therapies to market.

While labor and tariff reforms in the revised North American trade agreement may have more visible impacts on the United States economy, the final document levels a major blow to exclusivity and patent protections important to innovator biotech and pharma companies.

FDA has an official new leader, following a Senate vote to confirm Stephen Hahn as the agency’s next commissioner.
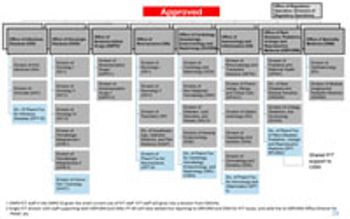
FDA’s Office of New Drugs restricting aims to improve scientific exchange and information sharing among review professionals.

A NASEM report stresses the importance of information sharing by biopharma companies and cooperation among regulatory authorities.

Problems in assuring reliable drug quality and supply dampens progress in bringing lifesaving therapies to market.

Developers of biosimilars have become dismayed with difficulties in gaining acceptance and reimbursement from the US healthcare system.

FDA readies more efficient oversight processes while advancing collaboration with Europe.

A recent report released by an FDA task force highlights the financial, manufacturing, and policy issues underlying the drug shortages of important prescription medicines in the United States.

Falsified documents and manipulated test results prompt warnings and investigations.

After months of planning and explaining, Janet Woodcock, director of the Center for Drug Evaluation and Research (CDER), finally gained approval for broad changes in its process and procedures for evaluating and approving new drugs.

Time is running out on Ned Sharpless’ term as FDA acting commissioner, generating much talk as the administration shows interest in naming a new head for the agency.

The multitude of health reform measures emerging on Capitol Hill has accelerated lobbying action across the board, along with campaign donations to candidates from all sides.

Industry and regulators seek global system that reduces regional differences.

New tools and policies aim to support more complex manufacturing processes.

FDA, Health and Human Services, and the Trump Administration back cheaper foreign drugs to cut pharma costs.

New guidance documents clarify production standards and processes for developing interchangeable biologic drugs.

The US Senate and the US Pharmacopeial Convention debate the necessity of monographs for biologics.

Congress is weighing in on drug pricing with a range of measures that differ in style and substance.

CDER’s KASA program seeks manufacturer data on drug attributes and risks to inform oversight.

FDA is supporting the development and marketing of OTC therapies, including CBD products, by proposing new rules and an expanded review office and promoting digital technologies.

FDA is examining and updating its programs for overseeing global operations and international affairs.

New agency leadership is pressed to promote innovation while addressing safety and quality issues.

CDER Director Janet Woodcock is finalizing this more streamlined approach process for evaluating new drugs to handle the surge in drug application submissions.

Sharpless will seek further solutions to the opioid crisis and work to reduce cigarette use in adults and kids.

FDA is moving to shift industry away from step-wise batch production.

Norman (Ned) Sharpless, director of the National Cancer Institute at the National Institutes of Health, will become FDA acting commissioner.