
Soaring opioid use creates challenges for new drug development and supply-chain control.

Jill Wechsler is BioPharm International's Washington Editor, jillwechsler7@gmail.com.

Soaring opioid use creates challenges for new drug development and supply-chain control.

Social media use raises questions about applying old standards to new information technology.

After months of anticipation, FDA issued guidances last month that outlines its recommendations for developing and approving biosimilar therapies.

More collaboration and expanded oversight aim to compel manufacturers to follow GMPs.

Pressure to approve new user fees opens the door to action on drug shortages, prices, and regulation.
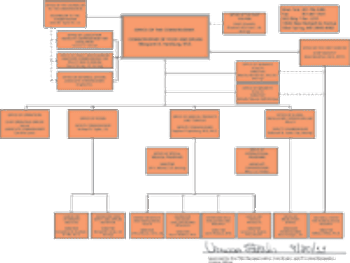
Added responsibilities and outside concerns prompt overhaul of agency's structure.

Clamor mounts over compromised care and rising costs due to lack of crucial therapies.

Manufacturers fund research and reduce prices to tackle diseases around the world.

PDUFA renewal legislation sets stage for new policies affecting revenue, research, and oversight.

Rising imports, overseas production spur collaboration and realignment of enforcement activities.

Follow-on versions of complex biologics require extensive expertise.

Industry struggles to curb drug abuse, diversion, and disruptions in supply to ensure access to quality products.

FDA, NIH, and industry seek new strategies to spur drug development and promote access to therapies.

Courts and Congress seek to reshape policies and programs affecting drug costs and access.

As drug shortages make headlines, FDA tests the Sentinel safety system and its effect on healthcare.

FDA prepares for top-level changes while promoting transparency and product safety

Top priorities for manufacturers include user fees, new health initiatives, and regulatory compliance.

Changes on Capital Hill create uncertainty for healthcare reform, drug regulation, and biomedical research.

FDA has to address multiple technical and legal issues to bring similar versions of biotech therapies to market.

Comparative effectiveness poses challenges for drug manufacturers.

A new strategy to streamline vaccine development and oversight.

Too many REMS cause headaches for doctors and the industry.

Plant closures, product recalls prompt FDA re-evaluation of GMP enforcement efforts.

More information may be available on drug approvals, prices, and research to expand public understanding of regulatory policies.

International outsourcing and rising theft spur regulatory action and manufacturer oversight.

Broader benefits and biosimilars will offset hefty fees and discounts while preserving R&D incentives.

The FDA is expanding postmarketing safety requirements, despite limited resources to manage these added responsibilities.

After months of increasingly rancorous debate, the House finally approved legislation March 21 that makes significant changes in the nation?s healthcare system.

The new Sentinel system aims to expand access to data on medical product safety and patient effects.

Trouble at Genzyme and with flu vaccine production illustrates the challenges in producing safe and potent biologics.