|Articles|December 15, 2019
Revision Process for Global/National Pharmacopoeias (PDF)
Author(s)J. Mark Wiggins, Joseph A. Albanese
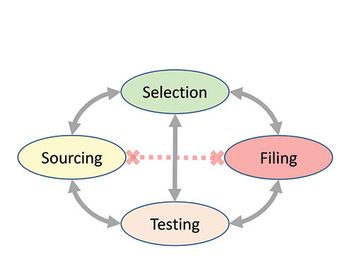
This article describes the revision process and the resulting publication of proposed and official updates for pharmacopoeias around the world.
Advertisement
Newsletter
Stay at the forefront of biopharmaceutical innovation—subscribe to BioPharm International for expert insights on drug development, manufacturing, compliance, and more.
Advertisement
Advertisement
Advertisement
Trending on BioPharm International
1
How CDMO Alliances Can Provide End-to-End Service that Reduces Drug Development Time and Costs
2
First-in-Human Study Validates Safety of Next-Generation mRNA–LNP Platform
3
FDA Clears PharmaResearch IND for Nano-Based Cancer Drug PRD-101
4
Industry Outlook 2026: Key Forces Transforming Drug Pipelines (Part 2)
5