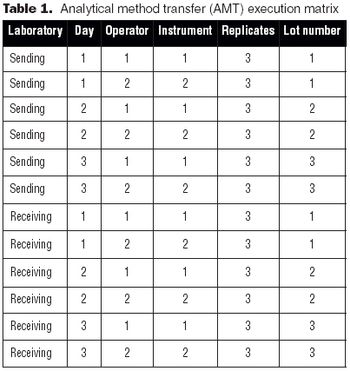
It is essential to understand the critical elements of validation extensions to ensure accurate process or product quality measurements.

It is essential to understand the critical elements of validation extensions to ensure accurate process or product quality measurements.
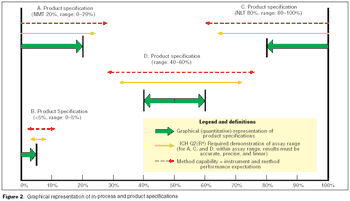
Validation of analytical methods can be more easily accomplished by breaking the task down into a series of planned steps.
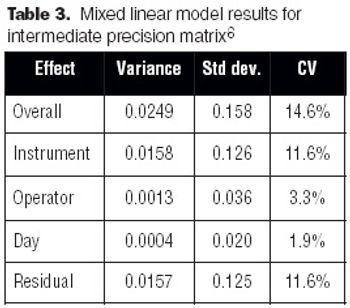
The development and optimization process can improve a method, but validation does not. Validation is the final proof that regulations and expectations are met.
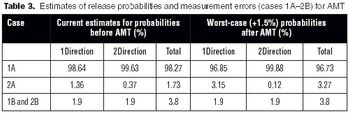
Several steps can be taken to maintain test method suitability after the formal completion of the analytical method validation studies,