
Biopharmaceutical companies are developing clever technologies for plant made pharmaceuticals (PMPs). When will the promise for the future turn into reality?

Biopharmaceutical companies are developing clever technologies for plant made pharmaceuticals (PMPs). When will the promise for the future turn into reality?

The last few months of 2004 were marked by crises likely to affect pharmaceutical and biotech manufacturing for years to come. Safety data indicated that antidepressants may cause problems for young patients that warrant black box warnings, new labeling, and special packaging. Merck pulled its blockbuster COX-2 inhibitor Vioxx off the market because studies showed increased risk of cardiac events from long-term use of the drug. To cap it off, British regulators shut down Chiron's Liverpool vaccine plant due to product contamination, creating a serious shortage of flu vaccine in the US.

Immune responses to foreign proteins are expected and may be anticipated for some self-proteins. The rapidity of development and the strength and persistence of the response depends on many factors.
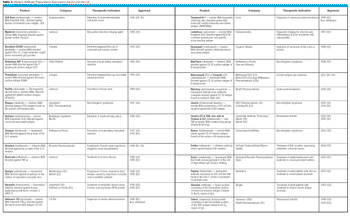
As of late 2004, 26 modern antibody-based therapeutic agents have been approved in the European Union and the US. Some 500 such products are currently in development, ensuring that the number of approved antibody-based products will increase substantially over the coming years.
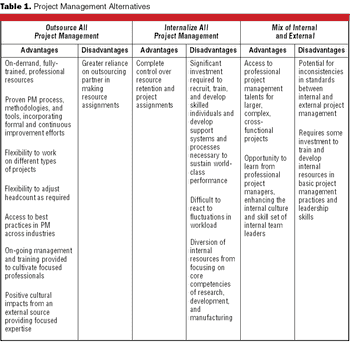
Project management is a specific set of skills and processes administered to meet the specific complexities of a project.

The holidays are upon us. It's the time of year that begins with gala parties in December and ends when you finally realize what you spent, usually around April 15 of the next year. Too Scrooge-like? Hope not. I just partook of some bad eggnog.