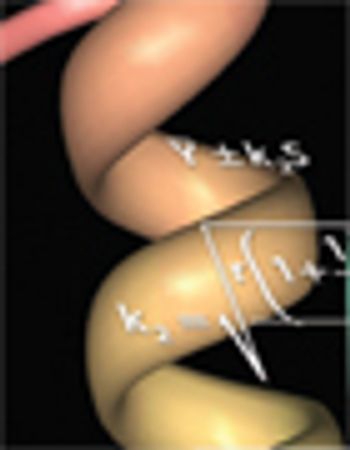
One goal of process characterization is establishing representative performance parameter ranges that can be used to set validation acceptance criteria (VAC). Characterization studies yield varying numbers of data points from multiple experiments, and may also include data generated at different scales (e.g., bench, pilot, and commercial), which add complexity to the analysis. Many statistical approaches can be used to set ranges from large data sets. As an example, we present the statistical considerations and techniques for setting validation acceptance ranges for a chromatography step used in purifying a recombinant protein. Performance parameter data from a combined data set consisting of 67 bench, six pilot, and three full-scale runs were analyzed using the statistical analysis software JMP (SAS Institute). The combined data set was used to compute tolerance intervals, so that sources such as scale and column feed material could be properly modeled. The resulting ranges were used to establish..