
Best Practices for Characterizing Formulation Design

Best Practices for Characterizing Formulation Design

Collaborations between Western and Indian companies may provide the best path for offshoring successfully and for developing India's readiness.
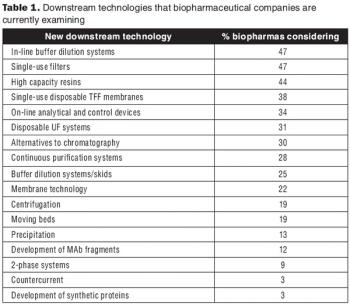
The new technologies being developed to improve downstream systems go beyond traditional chromatography.

An effective CAPA plan provides a mechanism for responding to the unexpected.
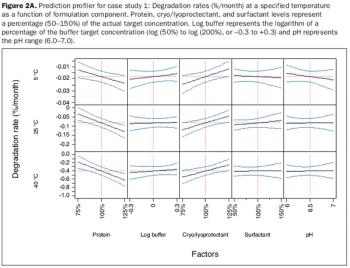
Design of experiments is a valuable tool for identifying aspects of a formulation that are critical to product quality.

The new Sentinel system aims to expand access to data on medical product safety and patient effects.
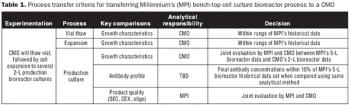
Sufficient process history is key to the rapid transfer of your process.

As a key element in the peptide production process, quality should be built into every step.

Sharing information is a critical part of security-whether we're protecting travelers from bombs or patients from adulterated medicines.
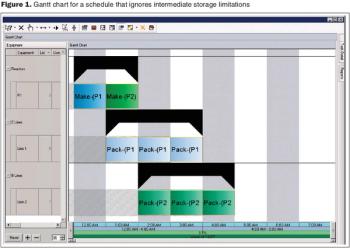
An algorithmic approach to fine tune facility design and predict capacity.